Regulation of Blood Glucose in the Body
Blood Glucose & Metabolic Adaptation in Starvation
Maintaining blood glucose levels within a normal range (70–110 mg/dL) is essential for homeostasis. The body regulates glucose levels through hormonal control, metabolic pathways, and organ-specific functions.
Several hormones control blood glucose by either increasing or decreasing its levels.
A. Hormones that Decrease Blood Glucose (Hypoglycemic Hormone)
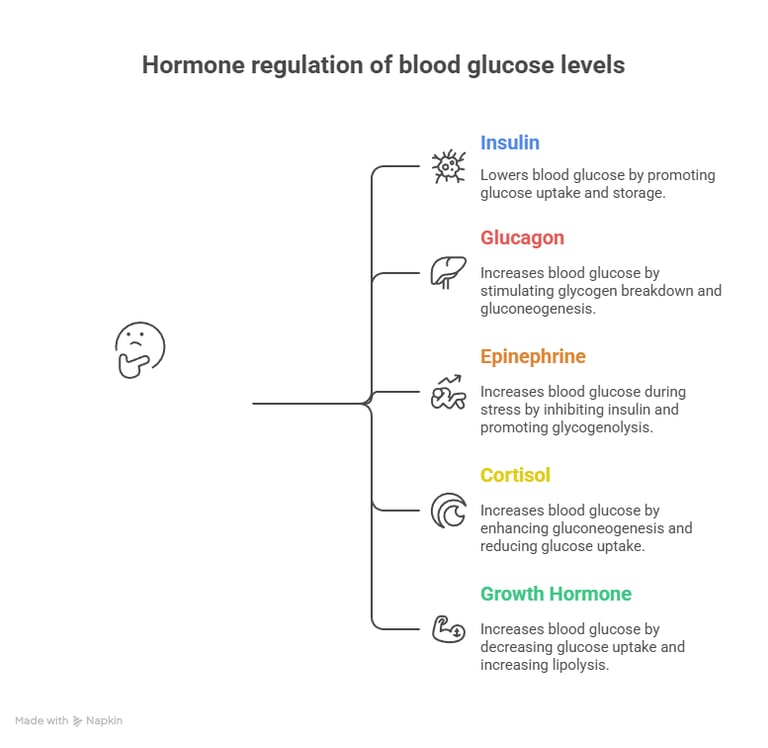
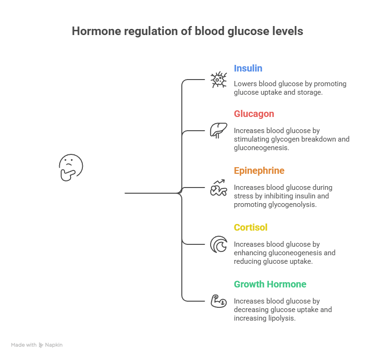
🔹 Insulin (Secreted by β-cells of the pancreas)
Promotes glucose uptake by cells (via GLUT4 in muscle & adipose tissue).
Stimulates glycogenesis (storage of glucose as glycogen in the liver & muscles).
Inhibits gluconeogenesis and glycogenolysis.
Enhances lipogenesis (conversion of glucose to fats).
Effect: Lowers blood glucose levels after a meal (Postprandial state).
B. Hormones that Increase Blood Glucose (Hyperglycemic Hormones)
1️⃣ Glucagon (Secreted by α-cells of the pancreas)
Stimulates glycogen breakdown (glycogenolysis) in the liver.
Promotes gluconeogenesis (formation of glucose from non-carbohydrate sources).
2️⃣ Epinephrine (Adrenaline) (From adrenal medulla)
Increases glycogenolysis in muscles and liver during stress.
Inhibits insulin secretion.
3️⃣ Cortisol (From adrenal cortex)
Enhances gluconeogenesis by breaking down proteins into amino acids.
Reduces glucose uptake by tissues.
4️⃣ Growth Hormone (GH) (From anterior pituitary)
Decreases glucose uptake by muscles and fat.
Increases lipolysis and gluconeogenesis.
Effect: Increases blood glucose levels during fasting, stress, or in case of energy demand.
2. Metabolic Pathways Controlling Blood Glucose
3. Organ-Specific Role in Blood Glucose Regulation
4. Clinical Conditions Related to Blood Glucose Regulation & Diabetes Mellitus
Summary
1. Hormonal Regulation of Blood Glucose
🍽️ 1. After Meals (Fed / Postprandial State)
🔹 Goal:
Utilize and store excess glucose.
Maintain plasma glucose within 70–110 mg/dL
🔹 Key Hormone: Insulin ↑
🌙 2. During Fasting (Post-absorptive Phase)
🔹 Goal:
Maintain normal blood glucose (for the brain, RBCs)
Mobilize stored fuel
🔹 Key Hormone: Glucagon ↑,
Insulin ↓
How the body maintains blood glucose homeostasis under three conditions:
A. Fed (post-meal), Fasting, and Starvation.
🕯️ 3. During Starvation (Prolonged Fasting, >2–3 days)
🔹 Goal:
Spare glucose & protein
Shift fuel use to fats and ketones
🔹 Hormones: High Glucagon, Cortisol, Epinephrine
Overview:
Maintaining normal blood glucose (~70–110 mg/dL) is a tightly coordinated process involving multiple organs — each with specific enzymes, transporters, and hormonal responses.
🔁Key regulators: Insulin, Glucagon, Cortisol, Epinephrine, and Growth Hormone.

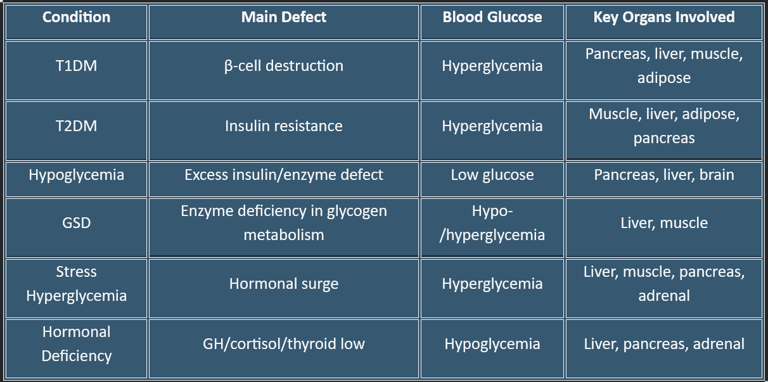
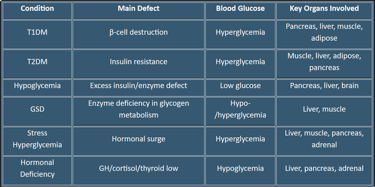
1. Diabetes Mellitus
a. Type 1 Diabetes (T1DM)
Cause: Autoimmune destruction of pancreatic β-cells → absolute insulin deficiency
Metabolic Effects:
Hyperglycemia due to lack of glucose uptake in muscle & adipose tissue
↑ Hepatic gluconeogenesis & glycogenolysis → further raises blood glucose
Lipolysis ↑ → ketone production → risk of Diabetic Ketoacidosis (DKA)
Organ Involvement: Pancreas (β-cell destruction), liver (excess glucose output), adipose (FFA release), muscle (impaired glucose uptake)
b. Type 2 Diabetes (T2DM)
Cause: Insulin resistance ± relative insulin deficiency
Metabolic Effects:
Muscle & adipose resist glucose uptake → hyperglycemia
Liver continues gluconeogenesis despite high glucose
Mild ketone production; rarely DKA unless stressed
Organ Involvement: Muscle, liver, adipose, pancreas
a. Liver – The Central Glucose Buffer (main Regulator)
Main Role: Maintains blood glucose by storing it after meals and releasing it during fasting/starvation.
Hormonal Regulation:
Insulin → activates glycogen synthase, inhibits glucose-6-phosphatase.
Glucagon → activates phosphorylase, PEPCK (for gluconeogenesis).
Outcome: Keeps blood glucose constant between meals.
b. Skeletal Muscle — Major Glucose Consumer
Main Role: Utilizes glucose for energy (ATP) and stores it as glycogen (but cannot release it back into the blood).
Note: the reason being
No glucose-6-phosphatase, so muscle glycogen cannot contribute to blood glucose directly.
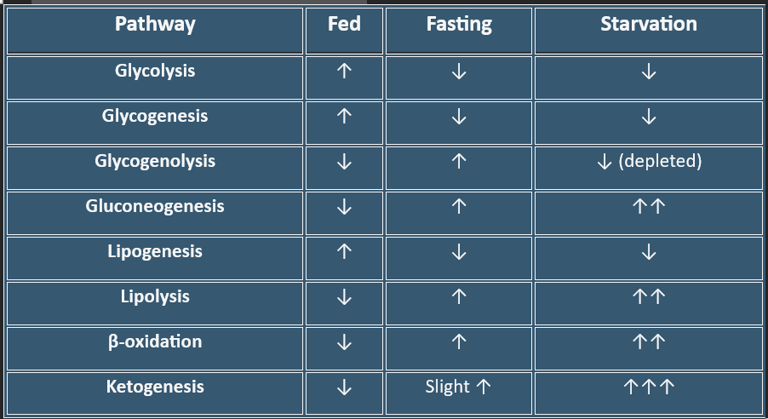
Summary Table — Metabolic Pathways by State
c. Brain — Glucose-Dependent Organ
Main Role: Continuous glucose user; cannot store or synthesize it.
Transport: GLUT3 (high-affinity transporter, insulin-independent)
Critical Point: Brain hypoglycemia → confusion, coma, irreversible damage.
d. Adipose Tissue — Energy Storage & Release
Main Role: Stores energy as triglycerides (fat); releases fatty acids during fasting for energy.
e. Pancreas — The Control Center (Hormonal Regulation)
Main Role: Senses blood glucose and releases hormones accordingly.
Balance:
Fed → Insulin dominates (Decreases Glucose)
Fasting → Glucagon dominates (Increases Glucose)
f. Kidneys — Gluconeogenesis & Reabsorption
Main Role: Supports blood glucose during prolonged fasting; prevents urinary glucose loss.
Filter excess glucose in diabetes/glucose appears in urine
Perform gluconeogenesis during prolonged fasting.
g. Small Intestine — Absorption Site
Main Role: Absorbs glucose after meals → delivers via portal vein to liver.
Transporters: SGLT1 (active), GLUT2 (facilitated)
Incretins (GLP-1, GIP) stimulate insulin release.
Key Integration Summary
Fed State:
Insulin promotes glucose uptake, storage, and utilization.
→ Pathways: Glycolysis, Glycogenesis, Lipogenesis.Fasting:
Glucagon maintains blood glucose via glycogenolysis and gluconeogenesis.Starvation:
Fatty acid oxidation & ketogenesis dominate.
→ Brain adapts to ketones, sparing glucose and protein.
Key Takeaways:
Liver = main regulator (buffer & glucose supplier)
Muscle = glucose consumer, glycogen storage
Brain = glucose priority; switches to ketones during starvation
Adipose = stores fat; releases FFA for energy & gluconeogenesis
Pancreas = hormonal controller (insulin/glucagon)
Kidney = secondary glucose production
Small intestine = nutrient absorption & incretin signaling
2. Hypoglycemia
Cause: Excess insulin, insulinoma, adrenal insufficiency, postprandial reactive hypoglycemia
Metabolic Effects:
Brain glucose deprivation → neuroglycopenic symptoms (confusion, seizures)
↑ Counter-regulatory hormones: glucagon, epinephrine → glycogenolysis, gluconeogenesis
Organ Involvement: Pancreas (insulin/glucagon), liver (glucose output), brain (glucose sensing)
3. Insulin Resistance & Metabolic Syndrome
Cause: Chronic overnutrition, obesity, sedentary lifestyle
Metabolic Effects:
Muscle/adipose resistant → decreased glucose uptake
Liver continues glucose production.
Hyperinsulinemia initially, later β-cell dysfunction → T2DM risk
Organ Involvement: Liver, muscle, adipose, pancreas
5. Hyperglycemia in Stress/Illness
Cause: Critical illness, surgery, trauma → ↑ cortisol, catecholamines, glucagon
Metabolic Effects:
Liver ↑ gluconeogenesis & glycogenolysis
Insulin resistance in muscle/adipose → hyperglycemia
Organ Involvement: Liver, muscle, pancreas, adrenal glands
4. Glycogen Storage Diseases (GSDs)
Cause: Genetic defects in glycogen metabolism enzymes
Examples:
Von Gierke Disease (GSD-I): Glucose-6-phosphatase deficiency → fasting hypoglycemia, hepatomegaly
McArdle Disease (GSD-V): Muscle glycogen phosphorylase deficiency → exercise intolerance
Organ Involvement: Liver, muscle
7. Pancreatic Disorders
Exocrine or endocrine tumors: Insulinoma → hypoglycemia; Glucagonoma → hyperglycemia, diabetes
Pancreatitis / Resection: Can lead to secondary diabetes (loss of β-cells)
6. Hypopituitarism / Hormonal Deficiencies
Cause: Deficiency of growth hormone, cortisol, thyroid hormone
Metabolic Effects:
↓ gluconeogenesis, glycogenolysis → fasting hypoglycemia
Organ Involvement: Liver, pancreas, adrenal glands
Key Takeaways:
Hyperglycemia is commonly due to insulin deficiency or resistance.
Hypoglycemia occurs due to excess insulin, enzyme defects, or hormonal deficiency.
The liver, muscle, adipose tissue, and pancreas are central organs; the brain is the primary consumer of glucose.
Other pathways:
- Stress Hyperglycemia → ↑ Cortisol, Catecholamines → Liver gluconeogenesis ↑
- Hormonal deficiencies (GH, Cortisol, Thyroid) → ↓ Glucose output → Hypoglycemia
- Brain → Critical glucose consumer in all conditions
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.