Essential Water-Soluble Vitamins
Support metabolism, immunity, and cellular functions with balanced intake of B-complex and vitamin C.
General Properties of Water-Soluble Vitamins
1. General Properties of Water-Soluble Vitamins
2. Recommended Daily Allowance (RDA)
✔ Solubility in Water – Easily dissolves in water-based fluids (blood, intracellular compartments).
✔ Limited Storage – Excess amounts are excreted in urine, thereby reducing the risk of toxicity.
✔ Frequent Dietary Need – Must be consumed regularly to prevent deficiency.
✔ Coenzyme Function – Many B vitamins act as enzyme cofactors in metabolism.
✔ Sensitive to Heat & Light – Can degrade during cooking or food processing.
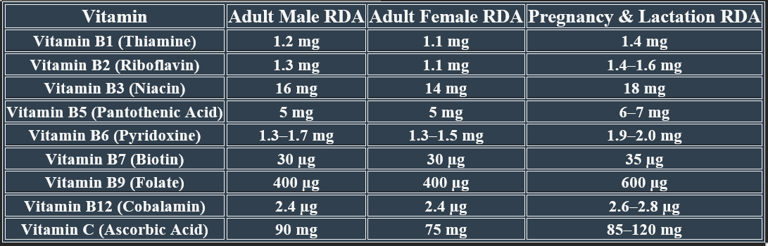
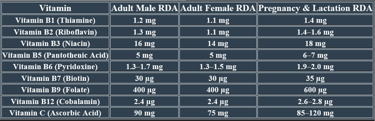
✅Higher needs during pregnancy/lactation for fetal development and maternal health.
✅ Deficiency risk increases with poor diet, malabsorption conditions, and aging.




Vitamin C is a powerful antioxidant essential for collagen synthesis, immune function, and iron absorption.
Since humans cannot synthesize it, daily intake from fruits and vegetables is crucial to maintain optimal plasma levels and prevent deficiencies.
Adults (Male/Female) ==== 90 mg / 75 mg
Pregnant Women ==== 85 mg
Lactating Women ==== 120 mg
✔ Higher intake needed in smokers and stressed individuals.
✔ Prolonged deficiency leads to scurvy (poor wound healing, bleeding gums).
1. Chemistry of Vitamin C
Molecular Formula: C₆H₈O₆
Structure: Contains a lactone ring with hydroxyl groups, allowing electron donation (antioxidant activity).
pH Sensitivity: Easily oxidized, sensitive to heat and light degradation.
✔ Acts as a reducing agent, neutralizing reactive oxygen species.
✔ Essential for enzymatic reactions in hydroxylation and collagen formation.
2. Biosynthesis of Vitamin C (Not in Humans)
✔ Synthesized in most animals and plants via the gulonolactone oxidase pathway.
✔ Humans lack gulonolactone oxidase, preventing endogenous synthesis.
✔ Dietary intake is required to maintain plasma levels.
3. Plasma Levels and Metabolism
✔ Normal Plasma Range: 0.4 – 1.7 mg/dL.
✔ Stored in leukocytes, adrenal glands, and brain tissue for functional use.
✔ Excess is excreted via urine, reducing toxicity risk.
Vitamin C (ascorbic acid) is a water-soluble antioxidant essential for collagen synthesis, immune support, and iron absorption. Humans cannot synthesize vitamin C, making dietary intake crucial.
3. Vitamin C: Chemistry, Biosynthesis, Plasma Levels
4. Recommended Daily Allowance (RDA)
5. Dietary Sources
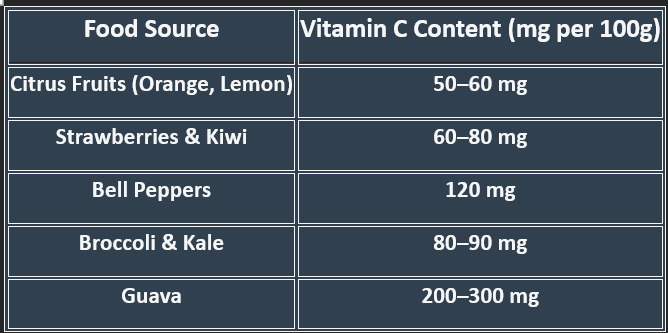
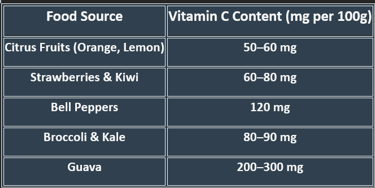
✔ Raw fruits & vegetables provide maximum vitamin C.
✔ Cooking reduces content, so minimal heat exposure is recommended.
Vitamin C plays a critical role in health by boosting immunity, reducing inflammation, protecting cells from oxidative damage, and supporting collagen formation. Let's explore how it interacts with diseases and nutrients.
6. Vitamin C in Disease Prevention & Nutrient Interactions
Role of Vitamin C in Disease Prevention
Vitamin C’s Interactions with Other Nutrients
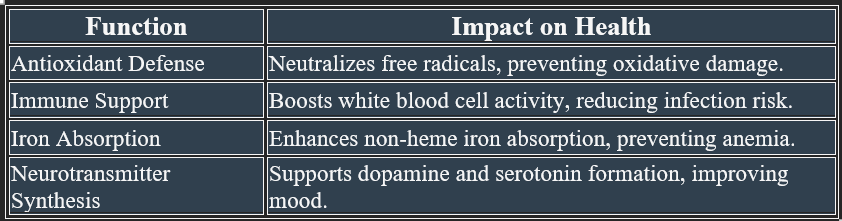
A. Immune Support & Infection Defense
✔ Enhances white blood cell function to fight infections.
✔ Reduces the severity and duration of colds and respiratory infections.
✔ Supports wound healing by increasing collagen synthesis.
B. Cardiovascular Health
✔ Protects against oxidative stress that contributes to heart disease.
✔ Improves blood vessel function by promoting nitric oxide production.
✔ Reduces LDL oxidation, lowering atherosclerosis risk.
C. Cancer Prevention
✔ Neutralizes free radicals, reducing DNA damage.
✔ Enhances iron absorption, supporting healthy cell function.
✔ Shows potential in high-dose intravenous therapy for cancer treatment (under research).
D. Neuroprotection & Cognitive Function
✔ Protects neurons from oxidative damage, reducing Alzheimer's disease risk.
✔ Improves neurotransmitter synthesis, supporting mood and mental health.
Vitamin C plays a critical role in health by boosting immunity, reducing inflammation, protecting cells from oxidative damage, and supporting collagen formation. Let's explore how it interacts with diseases and nutrients.




Vitamin C (ascorbic acid) plays critical roles in collagen synthesis, bile acid formation, antioxidant defense, immune function, and neurotransmitter production.
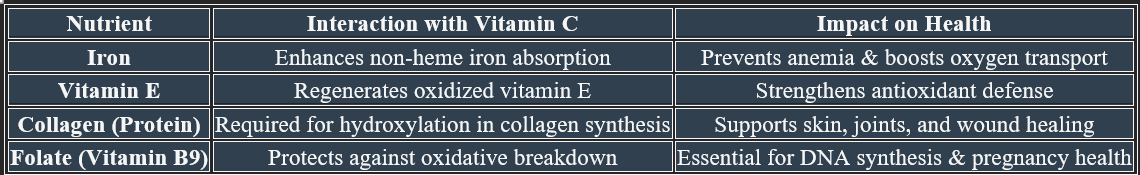
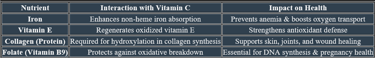
1. Role in Collagen Formation
✔ Essential for the hydroxylation of proline & lysine, stabilizing collagen structure
✔ Supports skin, cartilage, tendons, and blood vessels, ensuring tissue strength.
✔ Deficiency leads to scurvy, causing bleeding gums, fragile skin, and poor wound healing.
2. Role in Bile Acid Formation
✔ Vitamin C is required for cholesterol metabolism, leading to bile acid production.
✔ Helps convert cholesterol into bile acids, supporting fat digestion.
✔ Deficiency impairs bile synthesis, increasing cholesterol levels and gallstone risk.

7. Functions of Vitamin C
3. Other Important Roles


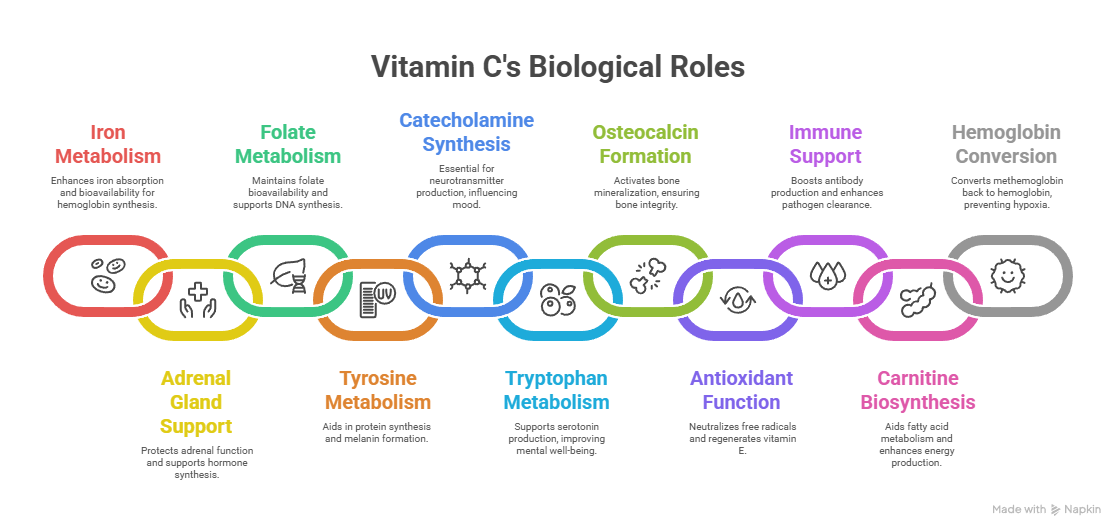
1. Iron Metabolism
✔ Enhances non-heme iron absorption in the intestine, preventing anemia.
✔ Reduces ferric (Fe³⁺) to ferrous (Fe²⁺) form, making it bioavailable for hemoglobin synthesis.
2. Role in the Adrenal Gland
✔ Concentrated in the adrenal cortex, supporting cortisol and stress hormone synthesis.
✔ Prevents oxidative damage, stabilizing adrenal function.
3. Folate Metabolism
✔ Protects folate from oxidative breakdown, maintaining its bioavailability.
✔ Supports DNA synthesis and methylation pathways, crucial for cell division.
4. Tyrosine Metabolism
✔ Required for hydroxylation of tyrosine, aiding in protein synthesis.
✔ Involved in melanin formation, supporting skin pigmentation and UV protection.
5. Synthesis of Catecholamines
✔ Essential for dopamine, norepinephrine, and epinephrine synthesis, influencing mood and stress response.
✔ Hydroxylates dopamine to norepinephrine, regulating neurotransmission.
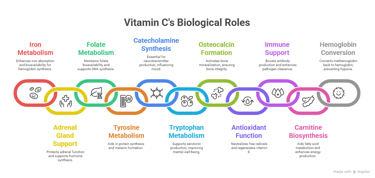
8. Vitamin C: Roles in Various Biological Processes
6. Tryptophan Metabolism
✔ Supports serotonin biosynthesis, improving mental well-being and sleep cycles.
✔ Reduces kynurenine accumulation, protecting against neuroinflammation.
7. Formation of Osteocalcin
✔ Activates osteocalcin, ensuring calcium binding for bone mineralization.
✔ Deficiency weakens bone integrity, increasing fracture risk.
8. Antioxidant Function
✔ Neutralizes free radicals, preventing oxidative damage.
✔ Regenerates vitamin E, ensuring continuous cellular protection.
9. Immunoglobulin Formation & Phagocytic Function
✔ Boosts B-cell immunity, supporting antibody production.
✔ Enhances neutrophil activity, improving pathogen clearance.
10. Carnitine Biosynthesis
✔ Required for hydroxylation reactions in carnitine formation, aiding fatty acid metabolism.
✔ Enhances mitochondrial energy production, reducing fatigue.
11. Conversion of Methemoglobin to Hemoglobin
✔ Reduces methemoglobin (Fe³⁺) back to hemoglobin (Fe²⁺), preventing hypoxia.
✔ Vital for oxygen transport and red blood cell function.
👉Final Take
Vitamin C is a powerful cofactor in metabolic pathways, essential for iron absorption, adrenal function, neurotransmitter synthesis, antioxidant defense, immune regulation, and oxygen transport. Its deficiency can lead to severe systemic dysfunctions, emphasizing its necessity for health.
9. Vitamin C Deficiency: Detailed Manifestations
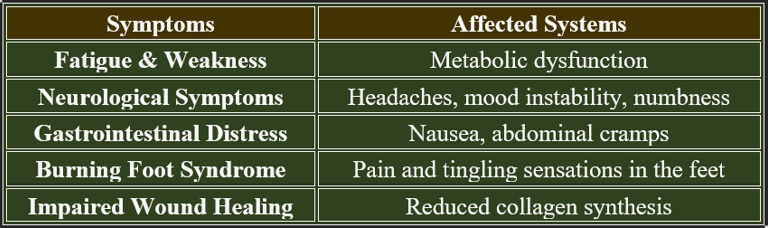
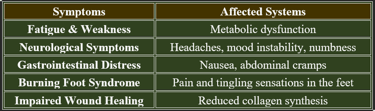
1. Early Signs of Vitamin C Deficiency
✔ Fatigue & Weakness – Due to impaired mitochondrial energy production.
✔ Poor Wound Healing – Reduced collagen synthesis weakens tissue repair.
✔ Easy Bruising – Capillary fragility increases bleeding risk.
✔ Dry Skin & Hair Loss – Collagen breakdown affects dermal health.
Vitamin C (ascorbic acid) is essential for antioxidant protection, immune function, collagen synthesis, and iron absorption. Its deficiency leads to serious systemic dysfunctions, the most severe form being scurvy.
2. Severe Deficiency: Scurvy
Scurvy occurs after prolonged deficiency (weeks to months). It leads to:
A. Skin & Mucosal Symptoms
✔ Petechiae & Purpura – Small red spots caused by ruptured capillaries.
✔ Gingival Swelling & Bleeding – Inflamed gums, potential tooth loss.
✔ Hyperkeratosis – Rough skin texture, follicular plugging.
B. Connective Tissue & Joint Manifestations
✔ Joint Pain & Swelling – Weak connective tissue causes arthritis-like pain.
✔ Muscle Degeneration – Loss of collagen weakens muscle integrity.
✔ Bone Fragility – Reduced osteoblast activity increases fracture risk.
C. Cardiovascular & Hematologic Effects
✔ Capillary Fragility – Leads to easy bleeding and internal hemorrhages.
✔ Anemia – Reduced iron absorption contributes to fatigue and pallor.
✔ Hypotension – Weak vascular integrity affects blood pressure regulation.
D. Neurological Symptoms
✔ Depression & Irritability – Affects neurotransmitter synthesis (dopamine, serotonin).
✔ Cognitive Decline – Memory impairment due to oxidative stress.
4. Prevention & Management
3. Risk Factors for Vitamin C Deficiency
✔ Malnutrition & Poor Diet – Low fruit and vegetable intake.
✔ Smoking & Alcoholism – Increased oxidative stress raises vitamin C demands.
✔ Gastrointestinal Disorders (IBD, Celiac Disease) – Impairs absorption.
✔ High Stress & Chronic Illness – Raises vitamin C requirements.
✔ Consume vitamin C-rich foods – Citrus fruits, berries, peppers, leafy greens.
✔ Supplementation for at-risk individuals – Helps prevent scurvy and oxidative damage.
✔ Address underlying conditions – Improve absorption and reduce oxidative burden.
👉Final Take
Vitamin C deficiency manifests as fatigue, poor wound healing, gum disease, joint pain, anemia, and neurological dysfunction. Maintaining adequate intake through diet or supplementation prevents severe complications, e.g., scurvy.
10. Hypervitaminosis C (Vitamin C Toxicity)
1. Causes of Hypervitaminosis C
✔ Excessive Supplementation – High-dose vitamin C intake beyond recommended limits.
✔ Renal Dysfunction – Impaired kidney function leading to accumulation.
✔ Prolonged High Intake – Chronic excessive consumption over time.


Vitamin C is water-soluble, meaning excess amounts are generally excreted in urine. However, high-dose supplementation (>2,000 mg/day) can lead to adverse effects.
2. Symptoms and Health Risks
3. Risk Factors
✔ Kidney Disease – Increased risk of oxalate stone formation.
✔ Genetic Disorders (G6PD Deficiency) – High-dose vitamin C may trigger hemolysis.
✔ Iron Storage Disorders (Hemochromatosis) – Excess vitamin C enhances iron absorption, increasing toxicity risk.
4. Management & Prevention
✔ Maintain recommended intake (75–90 mg/day for adults).
✔ Avoid excessive supplementation beyond 2,000 mg/day.
✔ Increase hydration to reduce kidney stone risk.
👉Final Take
While vitamin C is beneficial in moderate amounts, excessive intake can lead to gastrointestinal distress, kidney stone formation, and metabolic imbalances. Proper regulation of vitamin C intake ensures optimal health without toxicity risks.
11. Vitamin C: Interactions with Medications and Nutrient Absorption
Vitamin C plays a major role in nutrient metabolism, but it can also interact with medications, affecting their efficacy and side effects. Let’s explore its influence on drug interactions and nutrient absorption.
1. Interactions with Medications
2. Impact on Nutrient Absorption




✔ Boosts iron and folate bioavailability, but excessive amounts may disrupt calcium levels.
✔ Works with vitamin E to enhance antioxidant defense.
✔ Patients on blood thinners should moderate vitamin C intake to avoid clotting issues.
✔ Cancer patients should consult doctors before taking high-dose vitamin C.
👉Final Take
Vitamin C interacts with anticoagulants, chemotherapy drugs, and lipid regulators, which may impact treatment outcomes. It enhances iron and folate absorption but may alter calcium balance if overconsumed. Understanding these interactions ensures optimal health without adverse effects.
B-Complex group
a. Energy-releasing B vitamins
These act as coenzymes in carbohydrate, fat, and protein metabolism.
Vitamin B1 (Thiamine) – coenzyme as Thiamine pyrophosphate
Vitamin B2 (Riboflavin) – coenzyme as FMN and FAD
Vitamin B3 (Niacin / Nicotinic acid / Nicotinamide) – coenzyme as NAD⁺ and NADP⁺
Vitamin B5 (Pantothenic acid) – part of Coenzyme A
Vitamin B7 (Biotin) – a coenzyme in carboxylation reactions
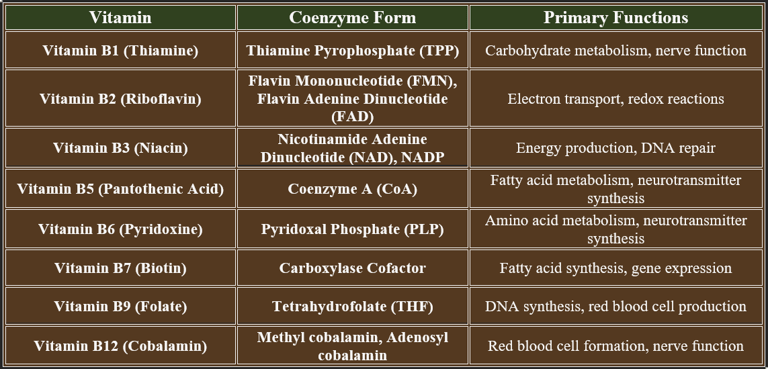
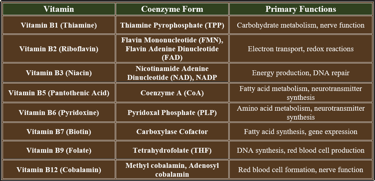
2. B-Complex Vitamins as Coenzymes
c. Other B vitamins (related to metabolism and growth)
Vitamin B6 (Pyridoxine, Pyridoxal, Pyridoxamine) – coenzyme as PLP (pyridoxal phosphate)
5. Causes of Water-Soluble Vitamin Deficiency
B-complex vitamins play critical roles in enzyme function, acting as coenzymes to support metabolic pathways, energy production, and biochemical reactions. Below is a summary of their coenzyme forms and functions:
1. Classification:
The B-complex vitamins are broadly classified into two functional groups & rest come under the third group:
a. Energy-releasing B vitamins
b. Hematopoietic (blood-forming) B vitamins
c. Other B vitamins (related to metabolism and growth)
b. Hematopoietic (blood-forming) B vitamins
These are essential for the synthesis and maturation of blood cells.
Vitamin B9 (Folic acid) – coenzyme as tetrahydrofolate
Vitamin B12 (Cobalamin) – coenzyme forms: Methyl cobalamin, deoxyadenosylcobalamin
The B-complex vitamins are a group of water-soluble vitamins that function mainly as coenzymes in various metabolic reactions, especially those involving the metabolism of carbohydrates, proteins, and fats.
They are essential micronutrients, not synthesized in adequate amounts in the human body, and therefore must be obtained from the diet.
✔ These coenzyme forms activate metabolic enzymes, ensuring proper biochemical function.
✔ They support ATP production, nervous system health, and cell division.
👉B-complex vitamins serve as essential coenzymes, driving metabolic reactions, energy synthesis, and cellular processes. Their deficiency leads to serious dysfunctions affecting metabolism, neurological health, and red blood cell formation.
✔ A balanced diet ensures sufficient intake and prevents deficiencies.
✔ Fortified foods help maintain optimal levels, especially for vegans and vegetarians.
4. Deficiency Symptoms of B-Complex Vitamins
3. Dietary Sources of B-Complex Vitamins
Adequate intake of B-complex vitamins is essential for metabolism, nervous system function, and red blood cell production. Deficiency leads to various health issues, affecting multiple body systems. B-complex vitamins are critical for cellular metabolism, neurological health, and red blood cell formation. Deficiency leads to serious health issues, emphasizing the need for a nutrient-rich diet and supplementation if necessary.
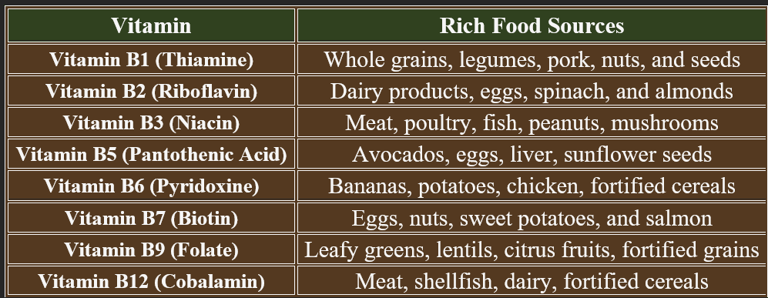
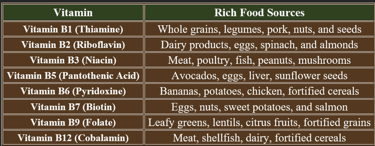
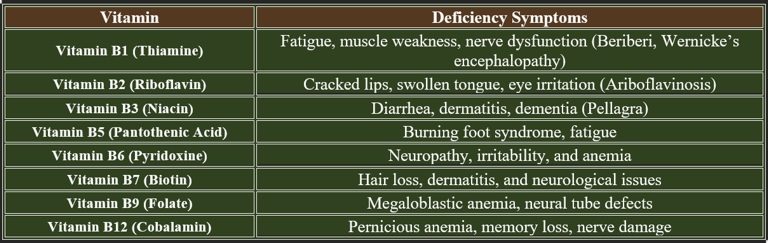

Water-soluble vitamin deficiencies occur due to insufficient intake, impaired absorption, increased demand, or excessive loss. Below are the key causes for deficiencies in B-complex vitamins
1. Dietary Deficiency (Inadequate Intake)
✔ Malnutrition – Poor dietary habits or restricted food consumption.
✔ Low Intake of Fresh Fruits & Vegetables – Leads to vitamin C deficiency.
✔ Vegan/Vegetarian Diets Without Fortification – Risk of B12 deficiency, as it is found primarily in animal products.
2. Malabsorption Disorders
✔ Gastrointestinal Diseases – Conditions like Crohn’s disease, celiac disease, and irritable bowel syndrome (IBS) reduce nutrient absorption.
✔ Pancreatic Disorders – Affect B-vitamin metabolism and digestion.
✔ Post-Surgical Changes (Gastric Bypass) – Reduced ability to absorb B12 and other vitamins from food.
3. Increased Vitamin Demand
✔ Pregnancy & Lactation – Higher need for folate, B12, and vitamin C.
✔ Illness & Infections – Increase vitamin C consumption for immune function.
✔ High Physical Activity – Demands more B-complex vitamins for energy metabolism.
4. Excessive Loss of Vitamins
✔ Chronic Diarrhea or Vomiting – Leads to depletion of B-complex and vitamin C.
✔ Alcoholism – Impairs thiamine, folate, and B6 metabolism, leading to severe neurological complications.
✔ Diuretics & Medications – Cause excessive loss of water-soluble vitamins through urine.
👉Water-soluble vitamin deficiency arises from poor diet, malabsorption, increased physiological demand, and excessive loss. Maintaining balanced nutrition and addressing underlying health issues prevents deficiencies and supports overall well-being.
B-Complex group
1. Thiamine (Vitamin B1):
Thiamine (Vitamin B1) is an essential water-soluble vitamin required for energy metabolism, nerve function, and carbohydrate processing.
1. Chemistry of Thiamine
✔ Molecular Formula: C₁₂H₁₇N₄OS
✔ Structure: Contains a thiazole ring and pyrimidine moiety, forming the active coenzyme Thiamine Pyrophosphate (TPP).
✔ Water-Soluble & Heat-Sensitive: Can degrade with excessive cooking or processing.
2. Biosynthesis of Thiamine
✔ Synthesized by bacteria, fungi, and plants—humans must obtain it through diet.
✔ Gut microbiota may contribute small amounts, but not enough to meet daily needs.
✔ Requires phosphorylation to form active TPP, essential for enzyme function.
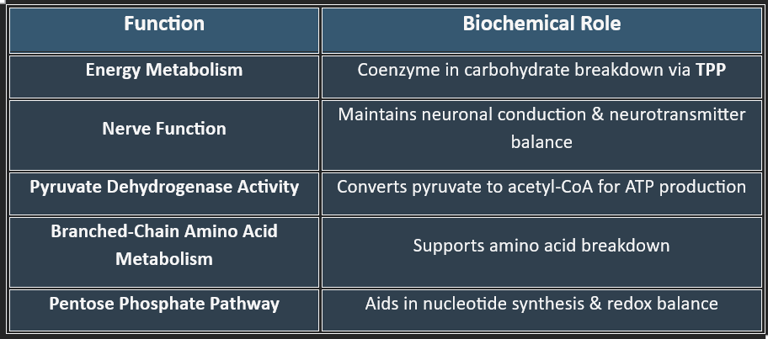
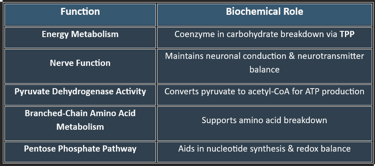
3. Functions of Thiamine
✔ Vital for ATP production, making it critical for metabolism and nerve health.
4. Plasma Levels of Thiamine
✔ Normal Range: 70–180 nmol/L
✔ Stored in the liver, skeletal muscle, and nervous system in small amounts.
✔ Excess is excreted via urine, reducing toxicity risk.
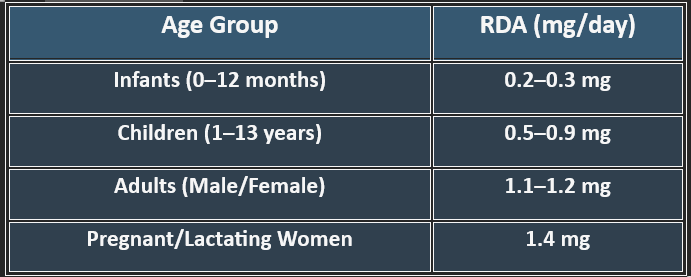
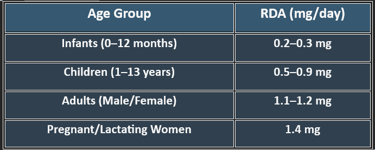
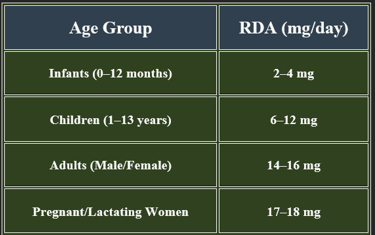
5. Recommended Daily Allowance (RDA)
✔ Higher needs during pregnancy, stress, and increased metabolic activity.
7. Deficiency Manifestations
✔ Common in alcoholics, malnourished individuals, and those with malabsorption disorders.
6. Dietary Sources of Thiamine
✔ Whole grains and lean meats are excellent sources of thiamine.
✔ Refined foods lose thiamine during processing, increasing deficiency risks.
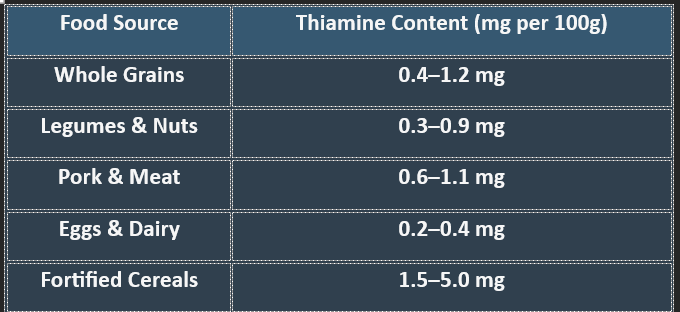
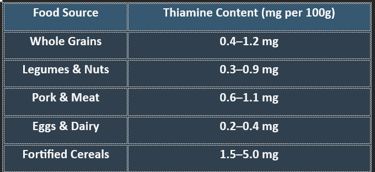
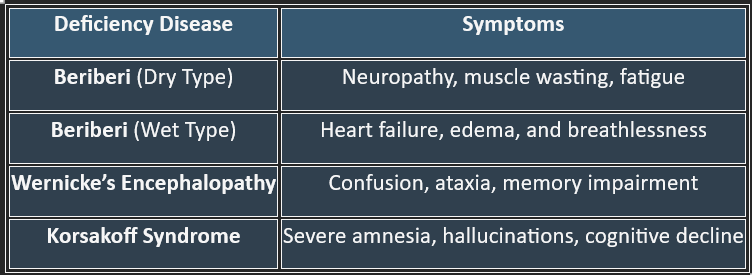
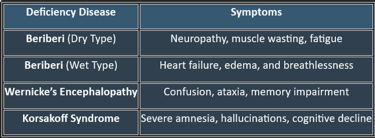
👉Thiamine is crucial for carbohydrate metabolism, nerve function, and ATP production. Deficiency leads to serious neurological and cardiovascular disorders, making adequate intake essential for overall health.
B-Complex group
2. Riboflavin (Vitamin B2):
Riboflavin (Vitamin B2) is a water-soluble vitamin essential for energy metabolism, cellular respiration, and antioxidant defense.
5. Recommended Daily Allowance (RDA)
✔ Increased demand during growth, pregnancy, and high metabolic activity.
1. Chemistry of Riboflavin
✔ Molecular Formula: C₁₇H₂₀N₄O₆
✔ Structure: Composed of a ribitol sugar linked to a flavin ring.
✔ Light-Sensitive: Easily degraded by UV exposure, making storage conditions critical.
2. Biosynthesis of Riboflavin
✔ Synthesized in bacteria, fungi, and plants, but not in humans.
✔ Humans must obtain it from dietary sources, making regular intake necessary.
✔ Converted into its active coenzyme forms:
Flavin Mononucleotide (FMN)
Flavin Adenine Dinucleotide (FAD)
3. Functions of Riboflavin
4. Plasma Levels of Riboflavin
✔ Normal Range: 5–25 nmol/L
✔ Stored in the liver, heart, and kidneys but quickly depleted without intake.
✔ Excess is excreted through urine, which is indicated by bright yellow coloration.
6. Dietary Sources of Riboflavin
✔ Dairy products and organ meats are excellent sources.
7. Deficiency Manifestations (Ariboflavinosis)
✔ Common in malnourished individuals, alcoholics, and those with absorption disorders.
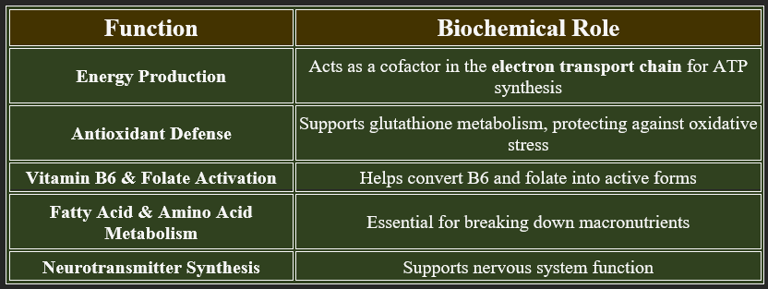

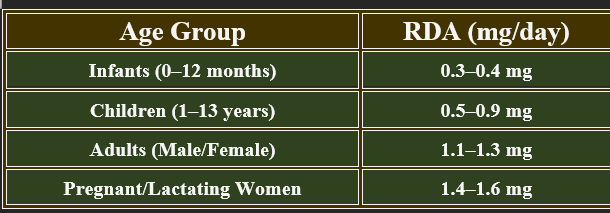
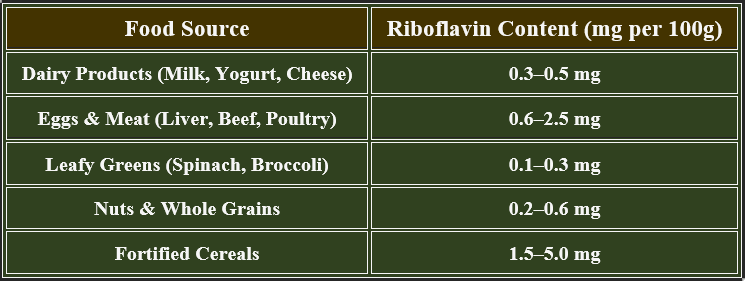

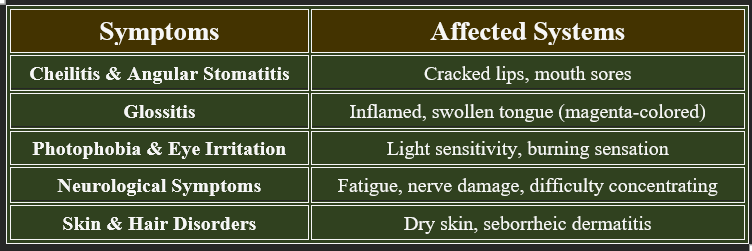

👉Riboflavin is essential for energy metabolism, enzyme activation, and antioxidant defense.
Deficiency leads to skin disorders, neurological dysfunction, and oral health issues. Regular dietary intake is crucial for maintaining optimal health.
B-Complex group
3. Niacin (Vitamin B3):
Niacin (Vitamin B3) plays a key role in energy metabolism, DNA repair, and cellular health. It exists in two forms: nicotinic acid and nicotinamide, both of which are precursors to the coenzymes NAD and NADP.
1. Chemistry of Niacin
✔ Molecular Formula: C₆H₅NO₂
✔ Structure: Pyridine ring with a carboxyl group (Nicotinic acid) or an amide group (Nicotinamide).
✔ Water-Soluble & Heat-Stable: Resistant to cooking losses.
✔ Active form: Converts to NAD (Nicotinamide Adenine Dinucleotide) & NADP (Phosphate form) for metabolic reactions.
2. Biosynthesis of Niacin
✔ Endogenous Synthesis from Tryptophan – 60 mg of tryptophan produces 1 mg of niacin.
✔ Requires Vitamin B6, Riboflavin, and Iron as cofactors in synthesis.
✔ Liver is the primary site for niacin conversion from tryptophan.
✔ Dietary intake is still necessary, as synthesis is limited.
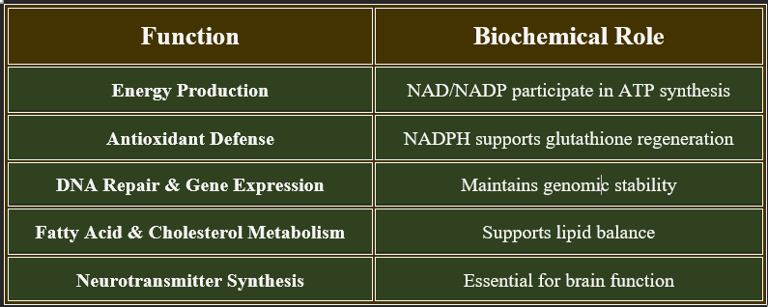
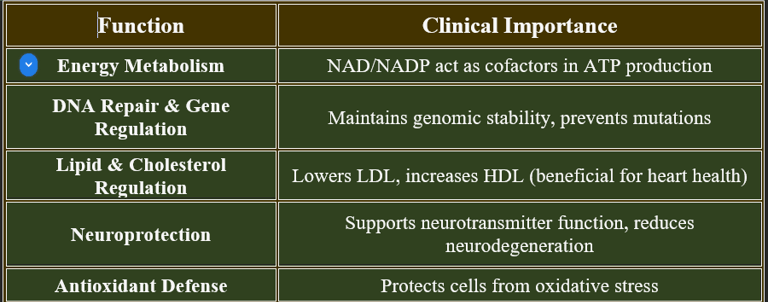
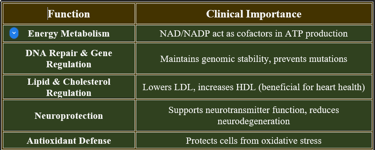
3. Functions of Niacin
✔ Vital for cellular metabolism, neurological health, and cardiovascular stability.
4. Plasma Levels of Niacin
✔ Normal Range: 0.5–8 µg/mL
✔ Stored in small amounts, primarily in the liver.
✔ Excess is converted into metabolic intermediates or excreted via urine.
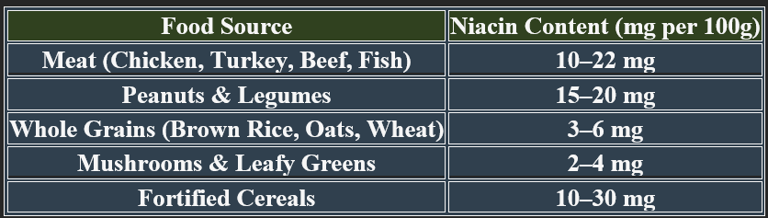
6. Dietary Sources of Niacin
✔ Animal-based foods contain preformed niacin; plant-based foods contribute via tryptophan conversion.
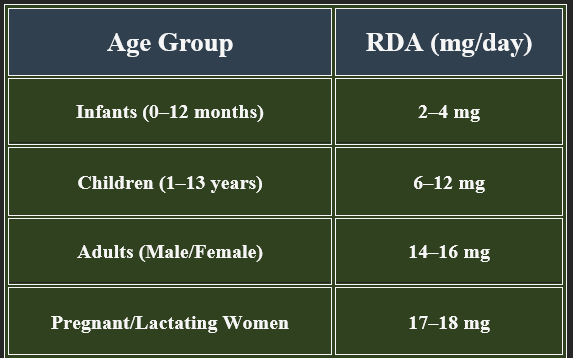
5. Recommended Daily Allowance (RDA)
✔ Higher requirements during pregnancy, metabolic stress, and high-energy expenditure.
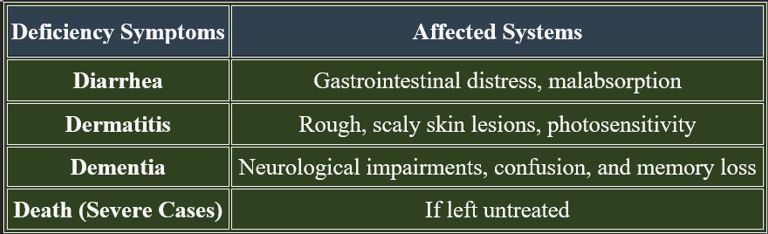
7. Deficiency Manifestations (Pellagra)
✔ Pellagra is common in malnutrition and impaired niacin metabolism.


👉Niacin is essential for cellular metabolism, neurological function, and antioxidant defense.
Deficiency leads to pellagra, manifesting as dermatitis, diarrhea, and cognitive decline. Adequate intake through diet and supplementation prevents deficiency complications.
Biosynthesis of Niacin
Niacin (Vitamin B3) is essential for cellular metabolism, DNA repair, and neurological function. It can be obtained through diet or synthesized endogenously from tryptophan.
1. Biosynthesis of Niacin from Tryptophan
✔ Precursor: Tryptophan (an essential amino acid)
✔ Conversion Efficiency: 60 mg of tryptophan produces 1 mg of niacin
✔ Key Enzymes Involved:
Tryptophan 2,3-Dioxygenase (TDO) – Initiates conversion in the liver.
Kynurenine Pathway – Intermediate stage producing quinolinic acid
Quinolinic Acid Phosphoribosyl Transferase (QPRT) – Converts quinolinic acid into niacin
✔ Requires Vitamin B6, Riboflavin, and Iron as cofactors.
✔ Impaired biosynthesis occurs in liver disease or B6 deficiency.
2. Biomedical Significance of Niacin
✔ Critical for metabolic stability, cardiovascular health, and cognitive function.
✔ Used in therapeutic interventions for dyslipidemia and neurological disorders.
👉Niacin is biosynthesized from tryptophan via the kynurenine pathway, requiring cofactor vitamins (B6, riboflavin) for optimal efficiency. Biomedical significance encompasses metabolic energy production, cardiovascular health, and neuroprotection, making it a vital nutrient.
Hypervitaminosis of Niacin (Vitamin B3 Toxicity)
1. Causes of Niacin Toxicity
✔ High-Dose Supplementation – Doses exceeding 500–2,000 mg/day.
✔ Extended-Release Niacin – Higher risk of liver toxicity compared to immediate-release forms.
✔ Medical Use for Cholesterol Management – Prescription-strength niacin may cause side effects.
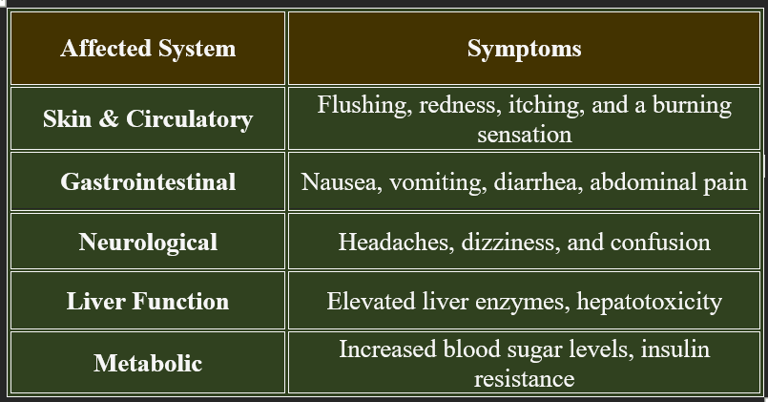
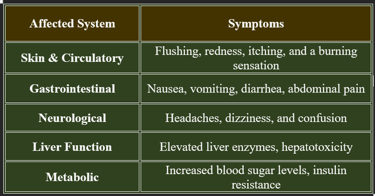
Niacin toxicity occurs when excessive doses of niacin are consumed, typically from high-dose supplements rather than food sources. While niacin is water-soluble, excessive intake can lead to adverse effects.
2. Symptoms of Niacin Toxicity
✔ Flushing is the most common symptom, occurring within 30 minutes of high-dose intake.
✔ Liver toxicity is a serious risk with prolonged excessive use.
3. Risk Factors for Niacin Toxicity
✔ Individuals taking high-dose niacin for cholesterol management.
✔ People with pre-existing liver disease or diabetes.
✔ Long-term use of extended-release niacin formulations.
✔ Monitoring liver function is essential for those on high-dose niacin therapy.
B-Complex group
4. Pantothenic Acid (Vitamin B5):
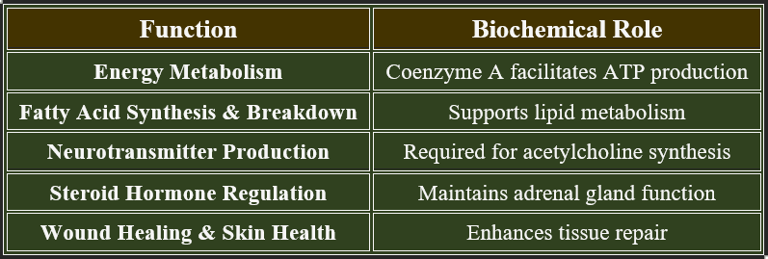
Pantothenic acid (Vitamin B5) is essential for energy metabolism, coenzyme A synthesis, and cellular repair. It supports fatty acid metabolism, neurotransmitter production, and adrenal function.
Pantothenic acid is essential for various biological processes. Chemically, it is composed of β-alanine and pantoic acid. It plays a crucial role in synthesizing coenzyme A (CoA), which is vital for cellular energy production and the metabolism of proteins, carbohydrates, and fats.
Pantothenic acid is stable under neutral conditions but can degrade in acidic or alkaline environments. Its calcium and sodium salts are more stable and are commonly used in supplements and animal feeds.
1. Chemistry of Pantothenic Acid
✔ Molecular Formula: C₉H₁₇NO₅
✔ Structure: Composed of pantoic acid linked to β-alanine.
✔ Water-Soluble & Heat-Sensitive: Can be degraded in food processing.
✔Biologically active form: Coenzyme A (CoA), required for metabolic functions.
2. Biosynthesis of Pantothenic Acid
✔ Synthesized in bacteria and plants, but not in humans—must be obtained through diet.
✔ Coenzyme A formation requires pantothenate kinase enzyme activity.
✔ Participates in the biosynthesis of fatty acids, neurotransmitters, and hormones.
✔ Essential for mitochondrial function and cellular respiration.
3. Functions of Pantothenic Acid
✔ Vital for enzyme function, cellular integrity, and hormone balance.
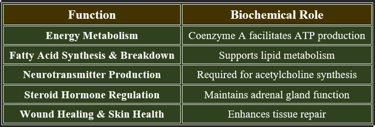
4. Plasma Levels of Pantothenic Acid
✔ Normal Range: 1–10 µg/mL
✔ Circulates primarily as Coenzyme A and free pantothenic acid.
✔ Excess is excreted via urine, reducing toxicity risk.
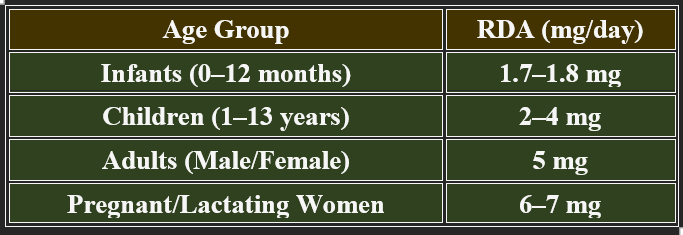
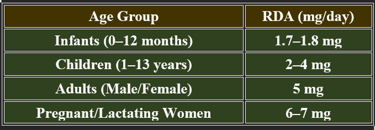
5. Recommended Daily Allowance (RDA)
✔ Increased demand during stress, pregnancy, and metabolic disorders.
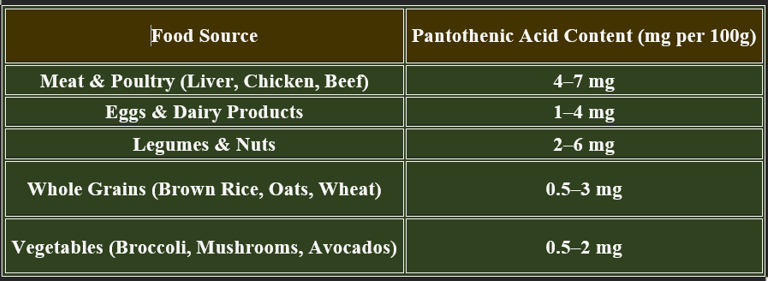
6. Dietary Sources of Pantothenic Acid
✔ Widely distributed in food sources, reducing deficiency risk.
7. Deficiency Manifestations (Hypo-pantothenosis)
✔ Rare in well-balanced diets but occurs in malnourished populations.

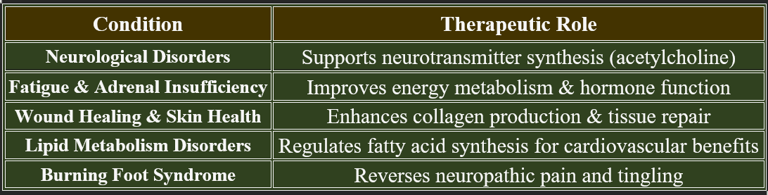
8. Therapeutic Applications of Pantothenic Acid
✔ Used in clinical settings to enhance metabolic stability and tissue regeneration.
✔ Higher doses may be beneficial in stress-related conditions.

B-Complex group
5. Pyridoxine (Vitamin B6):
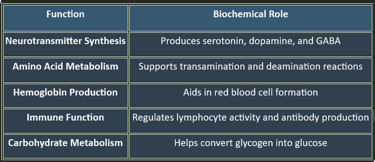
Vitamin B6 (Pyridoxine) is a water-soluble vitamin crucial for neurotransmitter synthesis, amino acid metabolism, and hemoglobin production. It exists in multiple forms, including pyridoxal, pyridoxamine, and pyridoxine, which convert to the active coenzyme pyridoxal-5-phosphate (PLP). Pyridoxine is critical for neurotransmission, metabolism, and immune regulation. Deficiency leads to neurological impairments, anemia, and skin disorders, emphasizing the importance of balanced dietary intake.
1. Chemistry of Pyridoxine
✔ Molecular Formula: C₈H₁₁NO₃
✔ Structure: Contains a pyridine ring with hydroxyl and methyl groups.
✔ Water-Soluble & Light-Sensitive: Degraded by heat and UV exposure.
✔ Active Form: Pyridoxal-5-phosphate (PLP), essential for enzymatic reactions.
2. Biosynthesis of Pyridoxine
✔ Synthesized by plants and microorganisms, but not by humans.
✔ Gut microbiota contributes minimally to B6 production.
✔ Requires phosphorylation to convert into its active coenzyme form (PLP).
✔ PLP participates in over 100 enzymatic reactions, making it essential for metabolism.
3. Functions of Pyridoxine
✔ Essential for brain function, cardiovascular health, and metabolic balance.
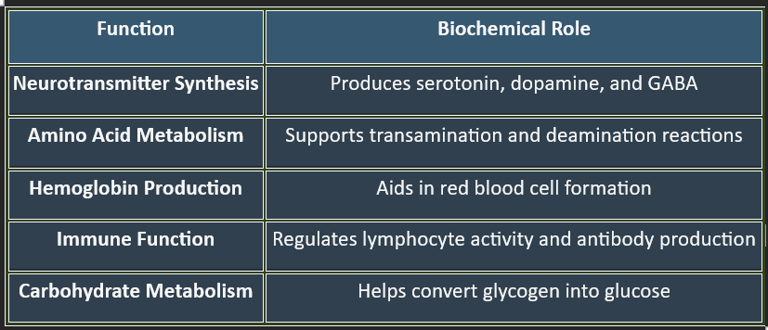
4. Plasma Levels of Pyridoxine
✔ Normal Range: 20–125 nmol/L
✔ Stored in muscle, liver, and brain, but excess is excreted via urine.
✔ PLP is the primary circulating form, used for metabolic assessments.
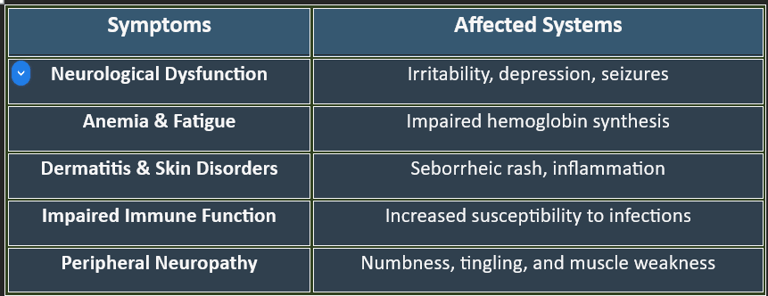
7. Deficiency Manifestations
✔ Common in alcoholics, elderly individuals, and those with absorption disorders.
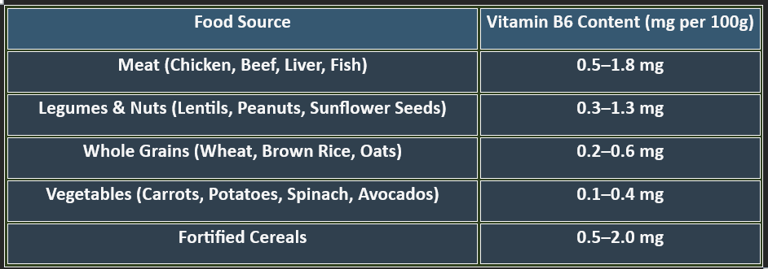
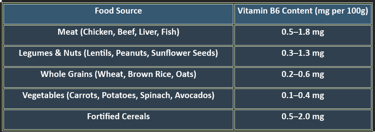
6. Dietary Sources of Pyridoxine
✔ Animal-based foods provide bioavailable pyridoxine, while plant-based sources contribute smaller amounts.
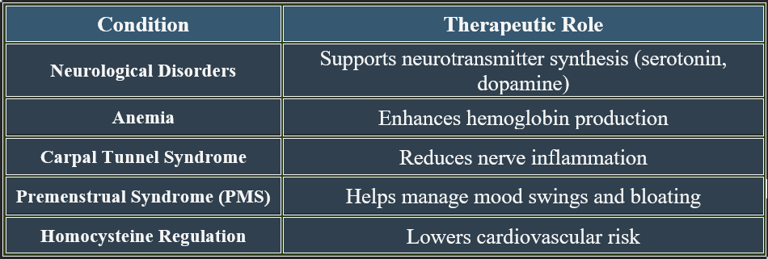
8. Therapeutic Applications of Pyridoxine
✔ Used in neurological, cardiovascular, and metabolic therapies.
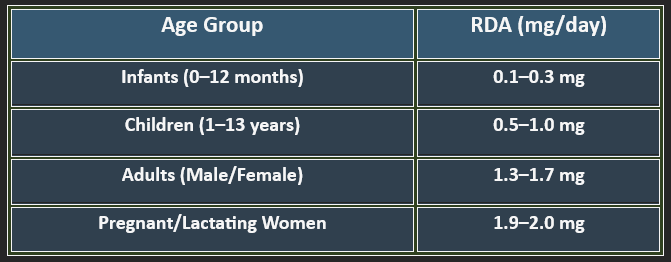
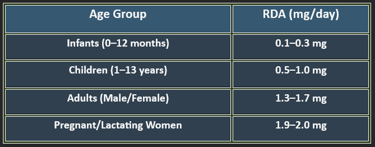
5. Recommended Daily Allowance (RDA)
✔ Higher needs during pregnancy, stress, and protein-rich diets.


B-Complex group
6. Biotin (Vitamin B7):
Biotin (Vitamin B7) is a water-soluble vitamin essential for carbohydrate, fat, and protein metabolism. It serves as a coenzyme in various enzymatic reactions, including DNA synthesis and cellular function.
1. Chemistry of Biotin
✔ Molecular Formula: C₁₀H₁₆N₂O₃S
✔ Structure: Contains a ureido ring fused to a thiophene ring.
✔ Water-Soluble & Heat-Stable: Resistant to cooking losses, but sensitive to strong acids and bases.
✔ Biologically Active Form: D-Biotin, required for enzymatic activity.
2. Biosynthesis of Biotin
✔ Synthesized by gut microbiota, but not sufficient to meet human needs.
✔ Produced in bacteria, fungi, and plants, requiring humans to obtain it from the diet.
✔ Cofactor for biotin-dependent carboxylases, essential for metabolic reactions.
✔ Absorbed in the small intestine via sodium-dependent multivitamin transporters (SMVTs).
3. Functions of Biotin
✔ Vital for growth, development, and metabolic regulation.
4. Plasma Levels of Folic Acid
✔ Normal Range: 3–15 ng/mL
✔ Stored mainly in the liver, with excess excreted via urine.
✔ Measured in diagnostics to assess nutritional status and anemia risk.
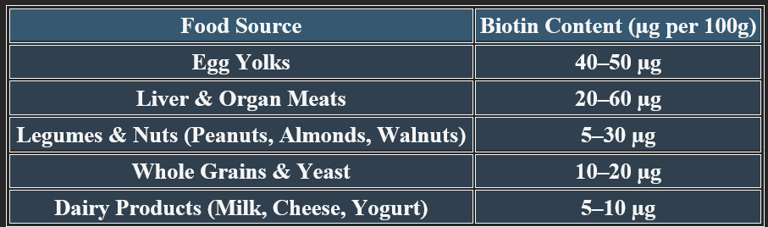
6. Dietary Sources of Biotin


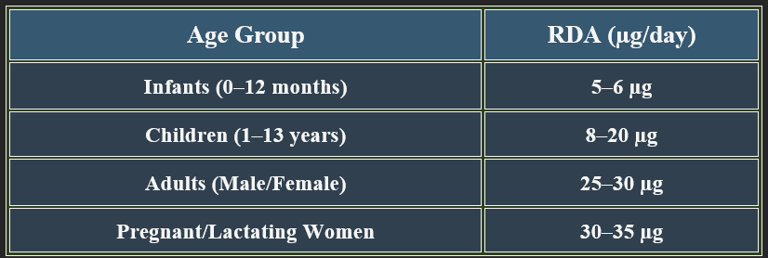
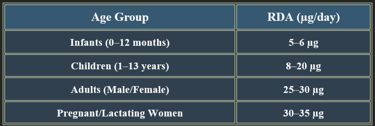
5. Recommended Daily Allowance (RDA)
✔ Higher needs during pregnancy to prevent neural tube defects.

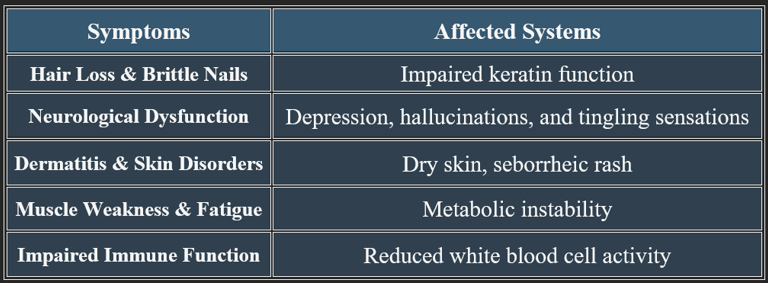
7. Deficiency Manifestations
✔ Rare in healthy individuals but occurs in those with genetic disorders or prolonged malnutrition.

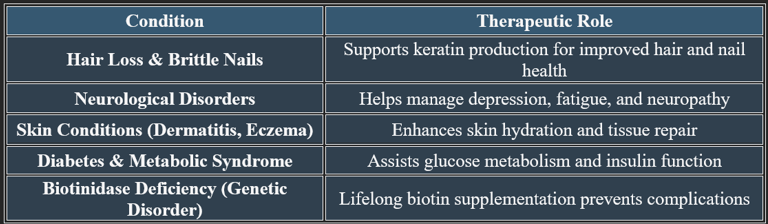
8. Therapeutic Applications of Biotin
✔ Used in dermatological, metabolic, and neurological therapies.

B-Complex group
7. Folic Acid (Vitamin B9):
1. Chemistry of Folic Acid
✔ Molecular Formula: C₁₉H₁₉N₇O₆
✔ Structure: Composed of pteridine ring, p-aminobenzoic acid (PABA), and glutamate residues.
✔ Water-Soluble & Heat-Sensitive: Easily degraded by cooking and oxidation.
✔ Biologically Active Form: Tetrahydrofolate (THF), required for metabolic reactions.
2. Biosynthesis of Folic Acid
✔ Synthesized by plants and bacteria, but not by humans—must be obtained through diet.
✔ Gut microbiota contribute minimally to folate production.
✔ Converted into its active coenzyme form, THF, via enzymatic reduction.
✔ Requires vitamin B12 for proper activation and function.
✔ Essential for nucleotide formation and DNA repair.
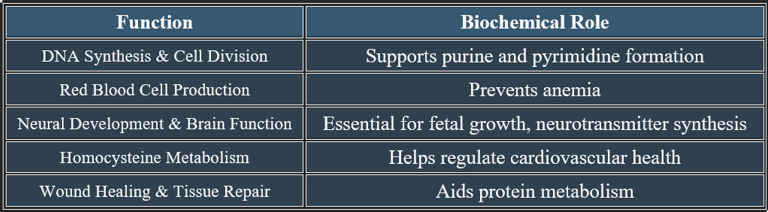
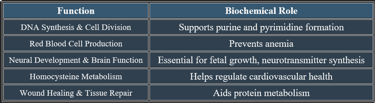
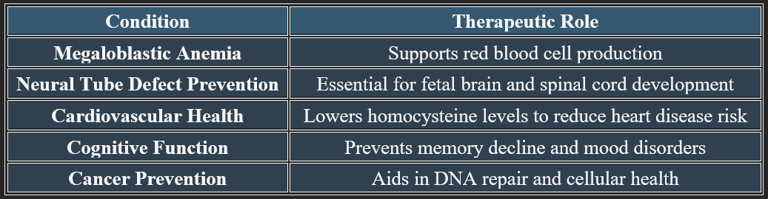
3. Functions of Folic Acid
✔ Vital for growth, development, and metabolic regulation.
Folic acid (Vitamin B9) is a water-soluble vitamin vital for DNA synthesis, red blood cell formation, and neural tissue development. It plays an important role during pregnancy and periods of rapid cell growth.
Folic acid is critical for DNA synthesis, brain function, and red blood cell production. Deficiency leads to anemia, cognitive impairment, and developmental disorders, emphasizing the importance of adequate dietary intake.
4. Plasma Levels of Folic Acid
✔ Normal Range: 3–15 ng/mL
✔ Stored mainly in the liver, with excess excreted via urine.
✔ Measured in diagnostics to assess nutritional status and anemia risk.
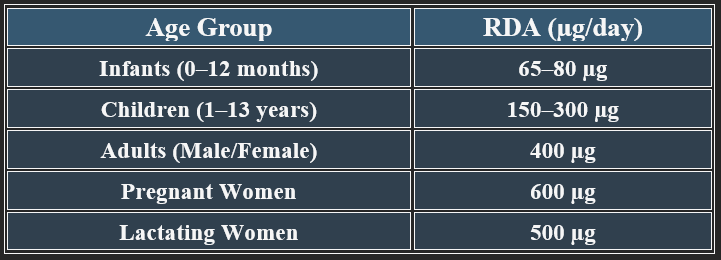
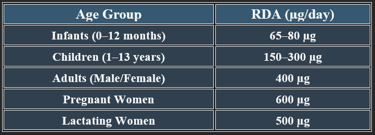
5. Recommended Daily Allowance (RDA)
✔ Higher needs during pregnancy to prevent neural tube defects.
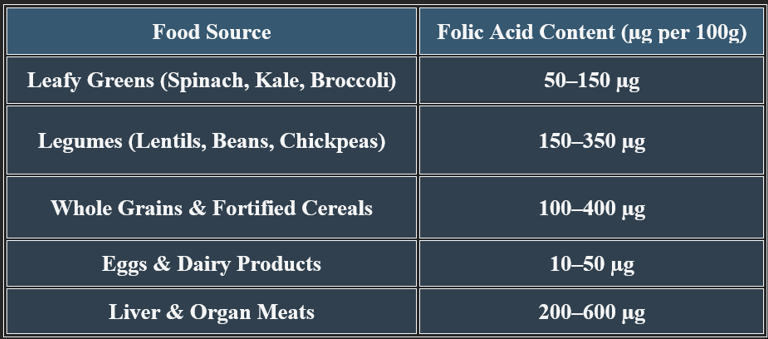
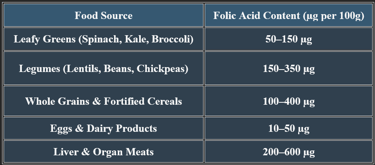
6. Dietary Sources of Folic Acid
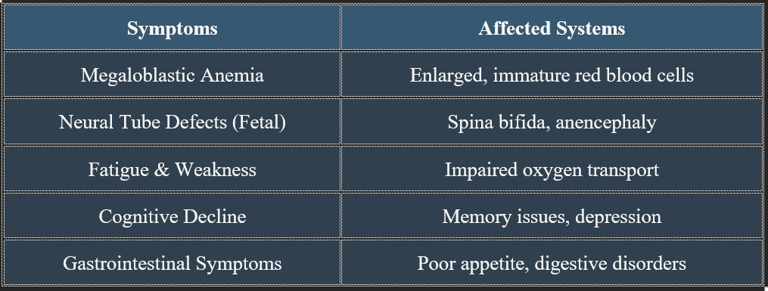
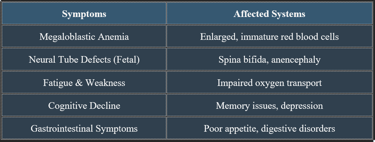
7. Manifestations of Folic Acid Deficiency
✔ Megaloblastic anemia is a key clinical hallmark of folate deficiency.
✔ Pregnant women with a folic acid deficiency are at risk of fetal developmental issues.

8. Therapeutic Applications of Folic Acid
✔ Folic acid is widely used in prenatal care to prevent birth defects.
✔ It plays a critical role in cardiovascular and neurological health.
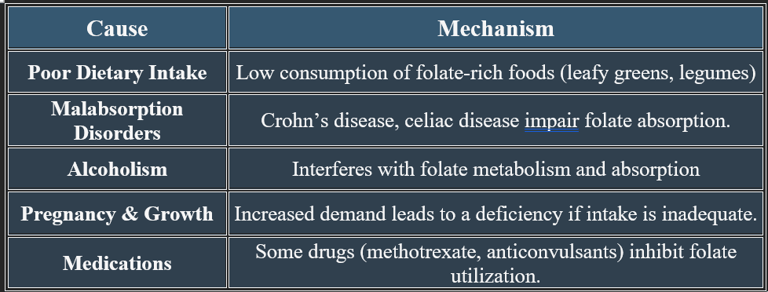
9. Causes of Folic Acid Deficiency
✔ Individuals with digestive disorders or excessive alcohol consumption are at high risk.
✔ Deficiency is common in populations with low folate intake or increased metabolic demands.

B-Complex group
8. Cobalamin (Vitamin B12):
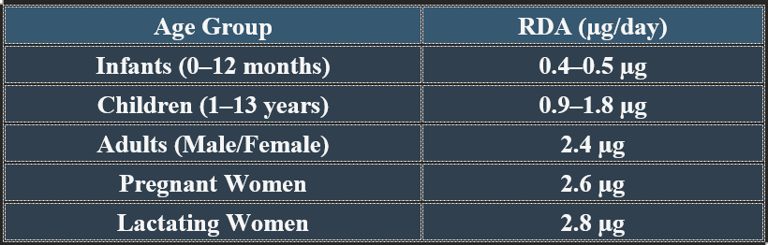
Cobalamin (Vitamin B12) is a water-soluble vitamin essential for DNA synthesis, red blood cell formation, and neurological function. It contains cobalt, distinguishing it from other B vitamins.
3. Functions of Cobalamin
Essential for brain health, blood formation, and metabolic stability.
1. Chemistry of Cobalamin
✔ Molecular Formula: C₆₃H₈₈CoN₁₄O₁₄P
✔ Structure: Complex corrin ring with a central cobalt ion.
✔ Coenzyme Forms:
Methyl cobalamin (Active in DNA & nerve function)
Adenosyl cobalamin (Essential for energy metabolism)
✔ Water-Soluble & Stable: Can be stored in the liver for years.
2. Biosynthesis of Cobalamin
✔ Synthesized by bacteria and archaea but not produced by plants or animals.
✔ Absorbed via intrinsic factor in the small intestine, ensuring efficient uptake.
✔ Converted into active forms (methyl cobalamin & adenosyl cobalamin) for biological use.
✔ Requires cobalt for its synthesis, making it unique among vitamins.
✔ Human intake depends on animal-derived or fortified foods.
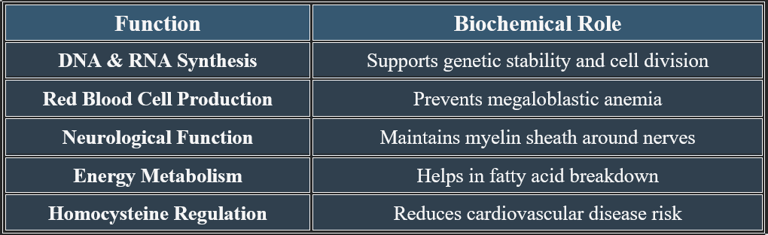

5. Recommended Daily Allowance (RDA)
✔ Higher needs in pregnancy, lactation, and older adults due to absorption decline.
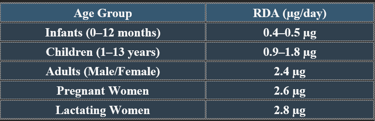
4. Plasma Levels of Cobalamin
✔ Normal Range: 200–900 pg/mL
✔ Stored primarily in the liver, with reserves lasting several years.
✔ Measured in diagnostics to assess anemia and neurological function.
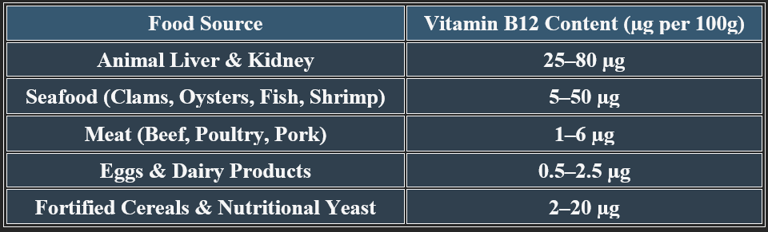
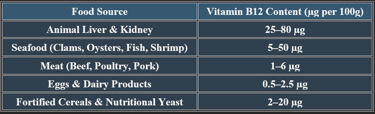
6. Dietary Sources of Cobalamin
✔ Animal products are primary sources; fortified foods support vegan diets.
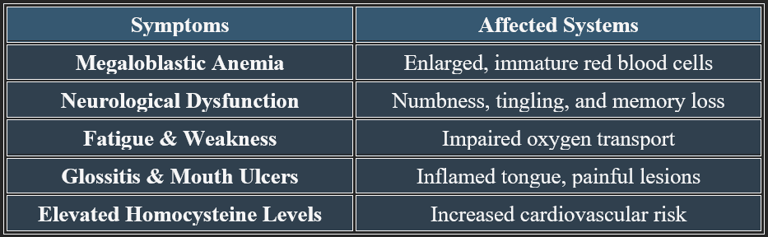
7. Deficiency Manifestations
✔ Deficiency is common in vegetarians, elderly individuals, and those with absorption disorders.
7a. Detailed Manifestations of Vitamin B12 Deficiency
✔ Untreated deficiency leads to irreversible nerve damage and cognitive decline.
✔ Megaloblastic anemia is one of the earliest clinical signs of B12 depletion.

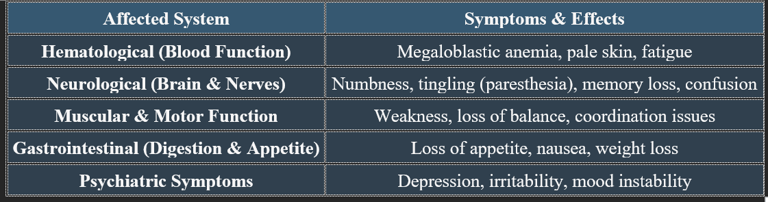

7b. Causes of Vitamin B12 Deficiency
✔ Deficiency is common in vegetarians, elderly individuals, and those with absorption disorders. Cobalamin is essential for blood formation, nerve function, and metabolic health. Deficiency leads to anemia, neurological impairments, and cardiovascular risks, emphasizing the need for adequate dietary intake.
8. Therapeutic Applications of Vitamin B12
✔ Used in neurological, cardiovascular, and hematological treatments.
Vitamin B12 deficiency is diagnosed through blood tests, metabolic markers, and anemia screening, while its therapeutic applications extend to neurological, cardiovascular, and hematological health. Adequate intake and supplementation ensure optimal function.
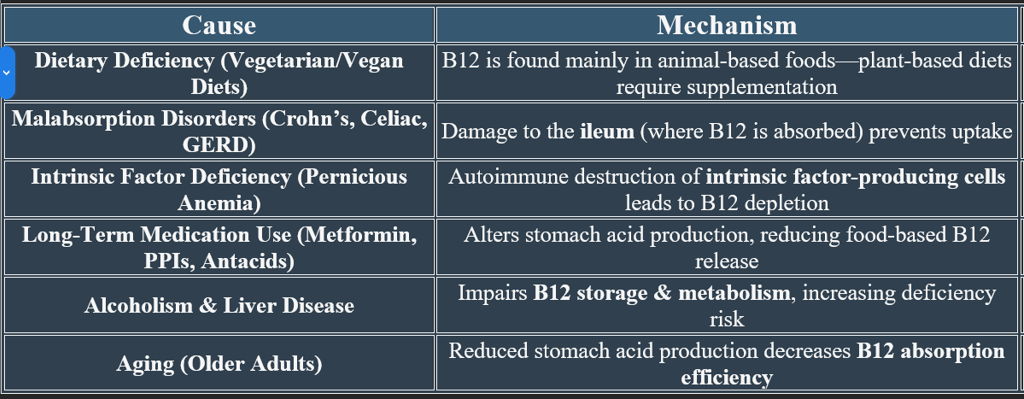

Vitamin B12 (cobalamin) requires a complex absorption process, including binding proteins and digestive enzymes. It also interacts with other vitamins and minerals, influencing metabolic pathways.
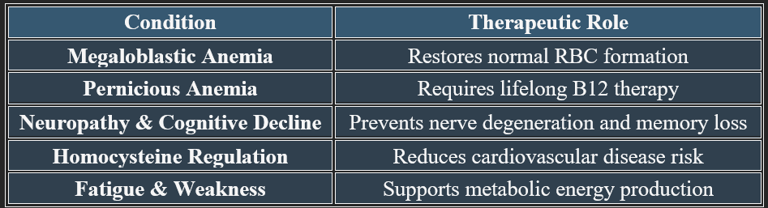

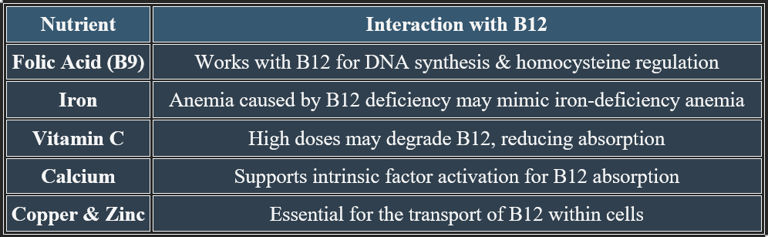

Vitamin B12 Absorption Mechanisms & Interactions with Other Nutrients
1. Mechanism of Vitamin B12 Absorption
✔ Step 1: Release from Food – Stomach acid liberates B12 from proteins in food.
✔ Step 2: Binding to R-protein (Haptocorrin) – Protects B12 from stomach acid degradation.
✔ Step 3: Transfer to Intrinsic Factor (IF) – In the small intestine, B12 binds to IF, a glycoprotein produced by parietal cells.
✔ Step 4: Absorption in the Ileum – The IF-B12 complex is absorbed in the last section of the small intestine (ileum).
✔ Step 5: Transport & Storage – B12 enters the bloodstream, binding to transcobalamin II, which delivers it to tissues.
👉Deficiency can occur due to IF dysfunction (e.g., pernicious anemia) or gastrointestinal malabsorption disorders.
2. Nutrient Interactions Affecting Vitamin B12 Metabolism
✔ Balanced intake of these nutrients enhances B12 function and prevents metabolic imbalances.
3. Factors Affecting B12 Absorption
✔ Low Stomach Acid (Hypochlorhydria) – Reduces protein-bound B12 release.
✔ Gastrointestinal Disorders (Celiac, Crohn’s Disease) – Impair ileal absorption.
✔ Pernicious Anemia – Autoimmune attack on intrinsic factor-producing cells.
✔ Alcoholism & Medication Use (Metformin, PPIs) – Interfere with absorption pathways.
✔ Monitoring B12 levels in individuals with digestive disorders helps prevent deficiency.
Neurological Applications of Vitamin B12
✔ Methylcobalamin is the preferred form for neurological therapies.
✔ Used in conjunction with folic acid and B6 for cognitive and nerve health.
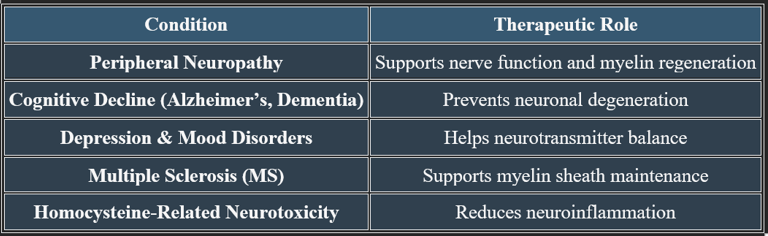

Vitamin B12 (Cobalamin) in Nerve Regeneration, Cognitive Protection, and Mental Stability
Vitamin B12 plays an essential role in neurological health, nerve regeneration, and cognitive function. It supports neurotransmitter balance, myelin sheath formation, and brain cell repair, making it crucial for mental well-being.
Vitamin B12 is vital for nerve regeneration, cognitive function, and emotional stability. It prevents neurological degeneration, supports neurotransmitter balance, and enhances mental clarity, making it essential for long-term brain health.
1. Role in Nerve Regeneration
✔ Myelin Sheath Maintenance – B12 helps produce and repair the myelin sheath, which insulates nerve fibers and supports signal transmission.
✔ Axonal Repair & Growth – Aids in nerve regeneration, reducing neuropathy symptoms such as tingling and numbness.
✔ Neurotransmitter Function – Supports neurotransmitter synthesis, improving nerve communication.
✔ Deficiency can lead to nerve damage, sensory disturbances, and coordination issues.
2. Cognitive Protection & Memory Function
✔ B12 plays a role in reducing oxidative stress and neuroinflammation.
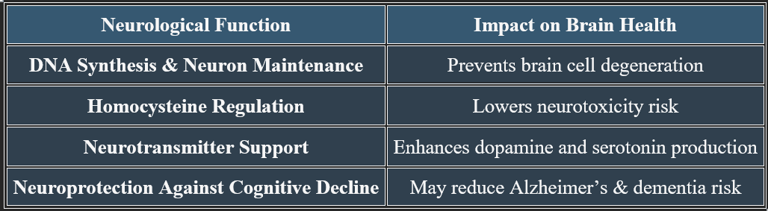

3. Role in Mental Stability & Mood Regulation
✔ Neurotransmitter Production – B12 supports serotonin and dopamine balance, reducing depression and anxiety.
✔ Brain Energy Metabolism – Enhances mental clarity, focus, and emotional stability.
✔ Hormonal & Stress Adaptation – Regulates the stress response by supporting adrenal function.
✔ Deficiency may lead to irritability, mood swings, and cognitive fog.
Absorption & Storage of Vitamin B12 (Cobalamin)
Vitamin B12 absorption is a complex process, involving protein carriers, digestive enzymes, and specialized transport mechanisms. Unlike other water-soluble vitamins, B12 can be stored in the liver, allowing long-term reserves.
1. Absorption Process of Vitamin B12
✔ Step 1: Release from Food – Stomach acid frees B12 from proteins in food sources.
✔ Step 2: Binding to R-protein (Haptocorrin) – Protects B12 from stomach acid degradation.
✔ Step 3: Transfer to Intrinsic Factor (IF) – In the duodenum, B12 binds to IF, a glycoprotein produced in the stomach.
✔ Step 4: Absorption in the Ileum – The IF-B12 complex is absorbed in the last section of the small intestine (ileum) via specific receptors.
✔ Step 5: Transport in Blood – B12 binds to transcobalamin II, delivering it to tissues.
👉 Intrinsic factor is essential for efficient absorption—its deficiency leads to pernicious anemia.
👉Absorption occurs mainly in the ileum, explaining why digestive disorders can lead to deficiency.
2. Storage & Distribution of Vitamin B12
✔ Primary Storage Site – Liver (~50–90% of total body B12).
✔ Other Tissues – Bone marrow, kidneys, nervous system.
✔ Stored in Active Forms – Methylcobalamin (for DNA & nerves) and Adenosyl cobalamin (for energy metabolism).
✔ Long-Term Reserve – B12 can be stored for years, unlike most other water-soluble vitamins.
✔ Stored B12 helps maintain metabolic stability during periods of low dietary intake.
3. Factors Affecting B12 Absorption & Storage
✔ Low Stomach Acid (Hypochlorhydria) – Impairs food-based B12 release.
✔ Gastrointestinal Disorders (Celiac, Crohn’s Disease) – Disrupt ileal absorption.
✔ Intrinsic Factor Deficiency (Pernicious Anemia) – Prevents B12 uptake.
✔ Alcoholism & Medications (Metformin, PPIs) – Interfere with absorption mechanisms.
✔ Deficiency can take months or years to manifest due to B12’s long-term storage capacity.
4. Factors That Impair B12 Absorption
✔ Low Stomach Acid (Hypochlorhydria) – Reduces protein-bound B12 release from food sources.
✔ Gastrointestinal Disorders (Crohn’s, Celiac Disease, GERD) – Damage to the ileum impairs absorption.
✔ Pernicious Anemia – Autoimmune attack on intrinsic factor-producing cells leads to lifelong B12 deficiency.
✔ Alcohol & Medications (Metformin, PPIs, Antacids) – Interfere with B12 metabolism.
✔ Vegan & Vegetarian Diets – Lack of animal-based B12 sources requires fortified foods or supplementation.
✔ Individuals at risk should consider routine B12 monitoring and supplementation.


B-Complex group
9. Vitamin-Like Substances: Inositol, Choline & PABA
Certain compounds, while not classified as essential vitamins, play critical roles in metabolism, brain function, and cellular health. Inositol, Choline, and Para-Aminobenzoic Acid (PABA) exhibit vitamin-like properties and are vital for various physiological processes.
Inositol, Choline, and PABA function as vitamin-like substances, contributing to brain health, metabolism, liver function, and skin protection. While not classified as essential vitamins, they play vital roles in overall health and well-being.
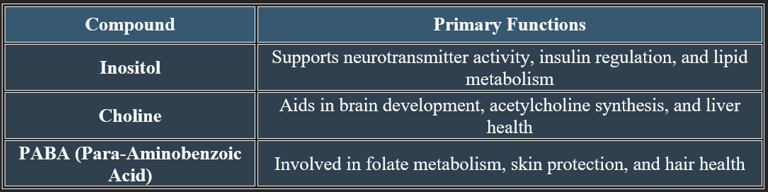
1. Inositol
✔ Chemical Structure: C₆H₁₂O₆ – A cyclic sugar alcohol.
✔ Function: Supports cell signaling, neurotransmitter activity, and lipid metabolism.
✔ Sources: Found in whole grains, citrus fruits, nuts, and legumes.
✔ Health Benefits:
Improves mental health (reduces anxiety & depression).
Enhances insulin sensitivity (supports glucose regulation).
Supports fat metabolism & liver function.
✔ Used in treating polycystic ovary syndrome (PCOS) and metabolic disorders.
2. Choline
✔ Chemical Structure: C₅H₁₄NO – An essential nutrient similar to B vitamins.
✔ Function: Key role in acetylcholine synthesis, liver health, and brain development.
✔ Sources: Found in eggs, meat, fish, soybeans, and cruciferous vegetables.
✔ Health Benefits:
Supports cognitive function & memory (required for neurotransmission).
Prevents fatty liver disease (aids lipid metabolism).
Essential for fetal brain development during pregnancy.
✔ Deficiency is linked to cognitive decline and liver dysfunction.
3. Para-Aminobenzoic Acid (PABA)
✔ Chemical Structure: C₇H₇NO₂ – A precursor to folic acid synthesis in bacteria.
✔ Function: Supports skin health, red blood cell formation, and metabolism.
✔ Sources: Found in liver, whole grains, eggs, and dairy.
✔ Health Benefits:
Used in skin treatments for sun protection.
Supports hair pigmentation & growth.
Involved in folate metabolism for DNA synthesis.
✔ Mostly used as a supplement in dermatological therapies.
Inositol, Choline, & PABA: Functions, Requirements & Deficiency Manifestations
1. Functions of Inositol, Choline, & PABA
✔ All three contribute to cellular communication, neurological stability, and metabolic balance.
2. Recommended Daily Intake
✔ Choline has an established RDA, while inositol and PABA are consumed based on dietary availability.
3. Deficiency Manifestations & Symptoms
✔ Deficiencies can affect mental health, metabolic function, and physical well-being.





BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
