Beta Oxidation
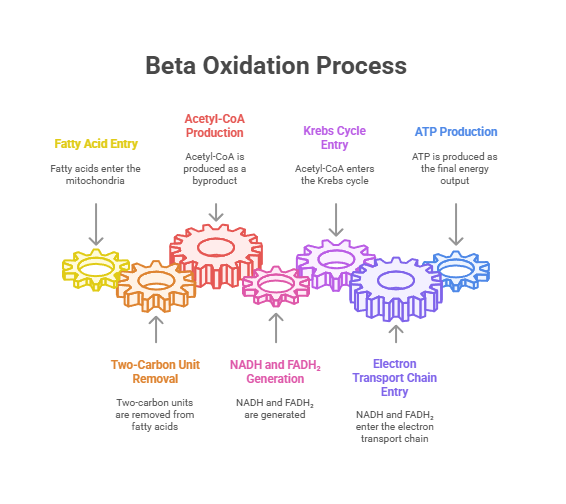
Beta-oxidation occurs in the mitochondria, where fatty acids are oxidized to generate ATP.
Activation: Fatty acids bind to Coenzyme A (CoA), forming Fatty Acyl-CoA.
Transport: Fatty Acyl-CoA enters mitochondria via the carnitine shuttle.
Beta-Oxidation: Sequential removal of 2-carbon units generates Acetyl-CoA, NADH, and FADH₂.
ATP Generation: Acetyl-CoA enters the Krebs Cycle, producing ATP via the Electron Transport Chain.
Beta oxidation is the biochemical process by which fatty acids are broken down in the mitochondria to generate ATP, the energy currency of the cell.
It involves sequential removal of two-carbon units from fatty acids, yielding Acetyl-CoA, NADH, and FADH₂, which then enter the Krebs cycle and electron transport chain to produce ATP.
1. Steps of Beta Oxidation
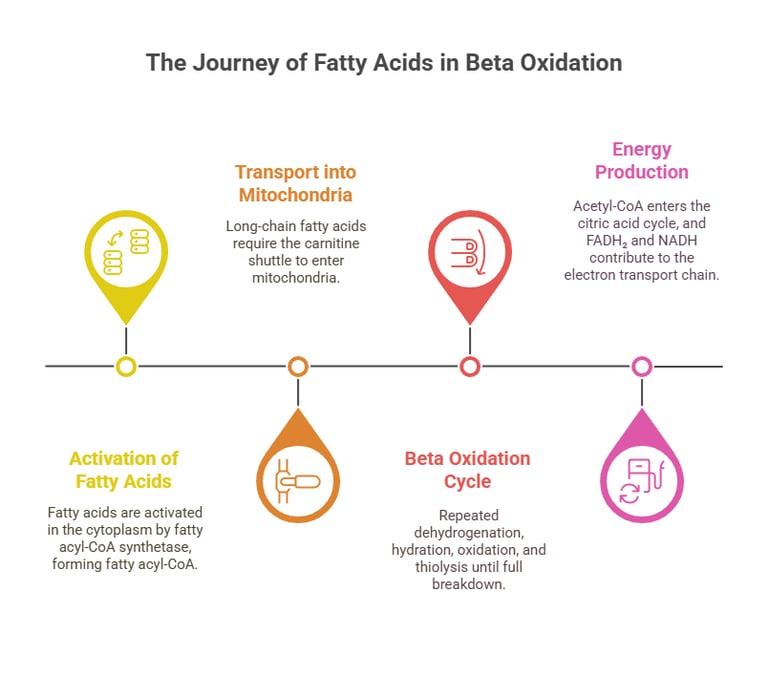
Beta oxidation occurs within the mitochondria and involves four key enzymatic steps that progressively break down fatty acids.
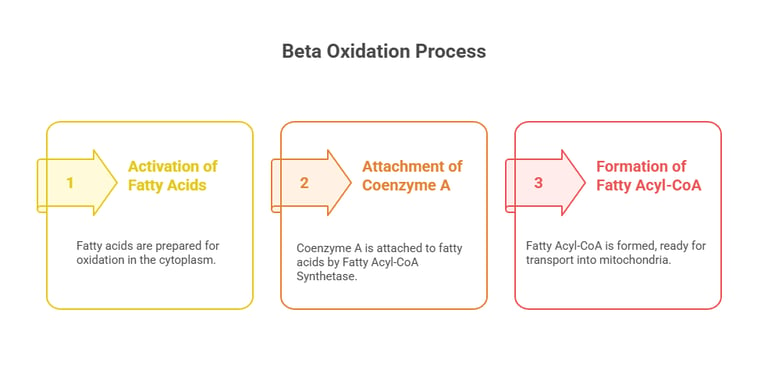
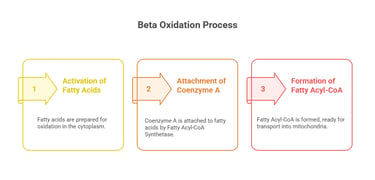
A. Activation of Fatty Acids (Cytoplasm)
Fatty acids must be activated before entering mitochondria.
Fatty Acyl-CoA Synthetase attaches Coenzyme A (CoA) to fatty acids, forming Fatty Acyl-CoA.
B. Transport into Mitochondria (Carnitine Shuttle)
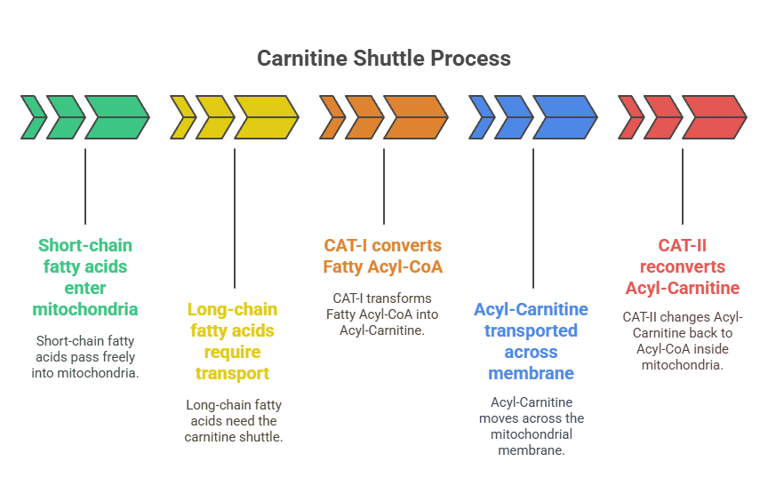
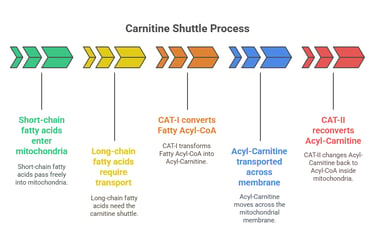
Short-chain fatty acids (<12 carbons) enter mitochondria freely, but long-chain fatty acids require transport via the carnitine shuttle.
Carnitine Acyltransferase I (CAT-I) converts Fatty Acyl-CoA into Acyl-Carnitine for transport across the mitochondrial membrane.
Carnitine Acyltransferase II (CAT-II) reconverts it back to Acyl-CoA inside the mitochondria.
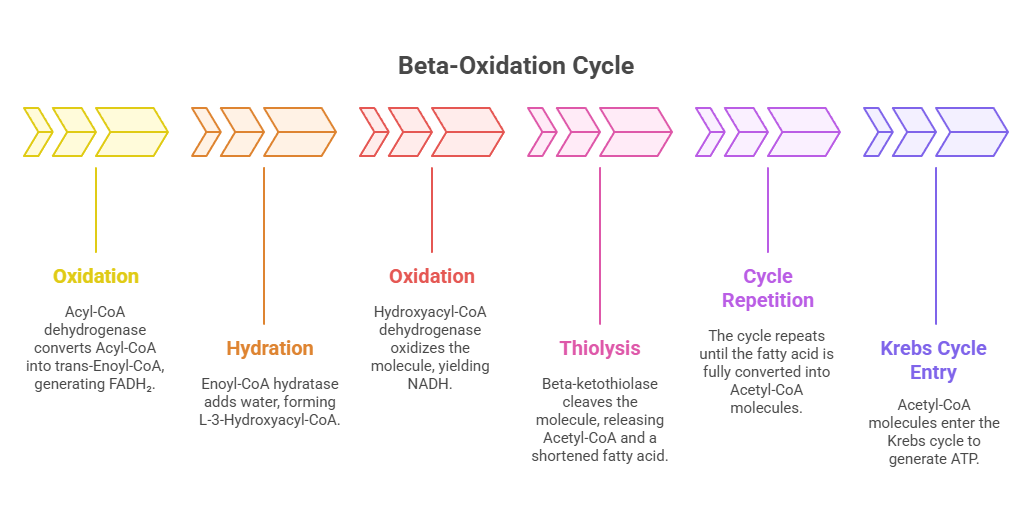
C. Beta-Oxidation Cycle (Inside Mitochondria)
Each cycle removes two-carbon units (Acetyl-CoA) from fatty acids:
Oxidation: Acyl-CoA dehydrogenase converts Acyl-CoA into trans-Enoyl-CoA, generating FADH₂.
Hydration: Enoyl-CoA hydratase adds water, forming L-3-Hydroxyacyl-CoA.
Oxidation: Hydroxyacyl-CoA dehydrogenase oxidizes the molecule, yielding NADH.
Thiolysis: Beta-ketothiolase cleaves the molecule, releasing Acetyl-CoA and a shortened fatty acid.
This cycle repeats until the fatty acid is fully converted into Acetyl-CoA molecules, which enter the Krebs cycle to generate ATP.
2. Energetics of Beta Oxidation
The ATP yield from beta oxidation is highly efficient, making fat an energy-dense fuel.
Example: Complete Oxidation of Palmitic Acid (C16)
Palmitic acid undergoes 7 cycles of beta oxidation:
7 NADH → 17.5 ATP
7 FADH₂ → 10.5 ATP
8 Acetyl-CoA → Enters Krebs cycle → 80 ATP
Total ATP Yield = 106 ATP per Palmitic Acid molecule
This makes fatty acid oxidation more efficient than glucose metabolism (which produces ~30-32 ATP per glucose molecule).
3. Clinical and Biological Significance of Beta Oxidation
B. Clinical Conditions Related to Beta Oxidation
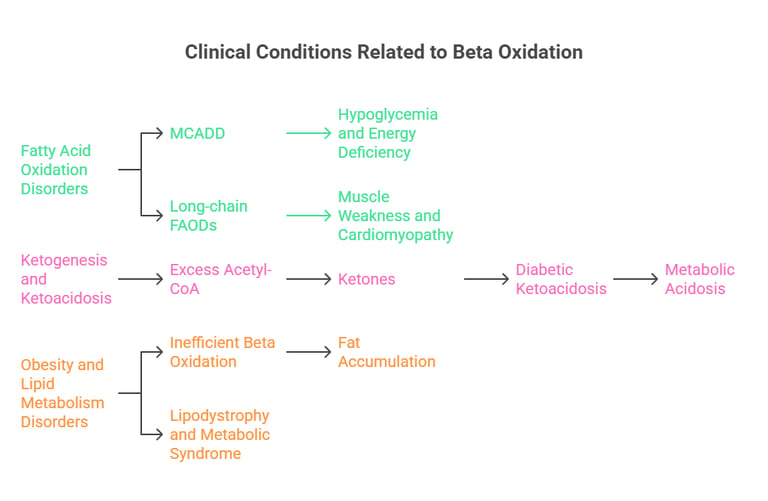
1. Fatty Acid Oxidation Disorders (FAODs)
Medium-chain acyl-CoA dehydrogenase deficiency (MCADD): Impaired beta oxidation leads to hypoglycemia and energy deficiency.
Long-chain fatty acid oxidation disorders: Defective carnitine shuttle results in muscle weakness and cardiomyopathy.
2. Ketogenesis and Ketoacidosis
Excess acetyl-CoA from beta oxidation is converted into ketones.
Diabetic ketoacidosis (DKA): Uncontrolled beta oxidation leads to excess ketone production, causing metabolic acidosis.
3. Obesity and Lipid Metabolism Disorders
In obesity, beta oxidation is inefficient, leading to fat accumulation.
Lipodystrophy and metabolic syndrome involve abnormal lipid utilization.
4. Regulation and Inhibition of Beta Oxidation
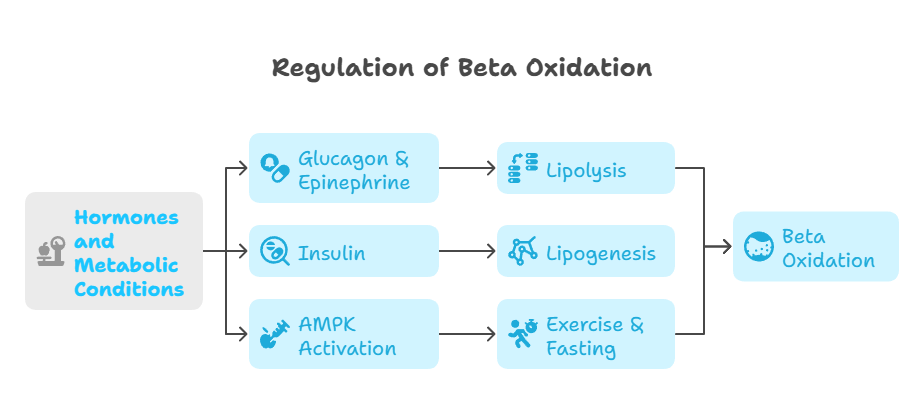
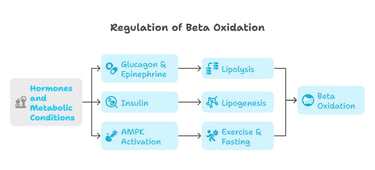
A. Regulation of Beta Oxidation
Hormones and metabolic conditions tightly control beta oxidation.
Glucagon & Epinephrine: Activate lipolysis, stimulating beta oxidation.
Insulin: Inhibits beta oxidation by promoting lipid storage (lipogenesis).
AMPK Activation: Enhances beta oxidation during exercise and fasting.
B. Inhibition of Beta Oxidation
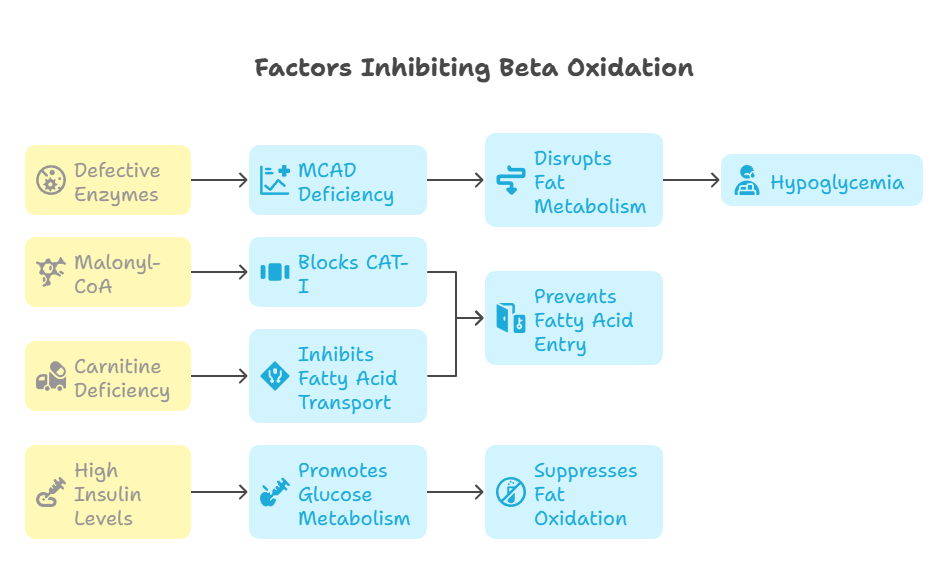
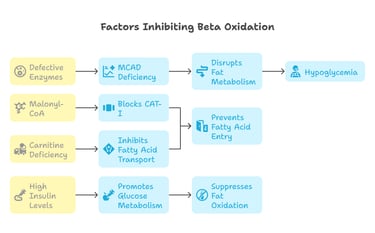
Certain factors inhibit beta oxidation:
Malonyl-CoA (Key Inhibitor):
Blocks Carnitine Acyltransferase I (CAT-I), preventing fatty acid entry into mitochondria.
Produced during lipogenesis, it prevents unnecessary fat breakdown when energy is sufficient.
Defective Enzymes:
MCAD Deficiency (medium-chain acyl-CoA dehydrogenase deficiency) disrupts fat metabolism, causing hypoglycemia.
Carnitine Deficiency:
Lack of carnitine inhibits fatty acid transport into mitochondria, impairing beta oxidation.
High Insulin Levels:
Insulin promotes glucose metabolism while suppressing fat oxidation, preventing beta oxidation activation.
5. Factors Affecting Beta Oxidation
Several biological and external factors impact beta oxidation efficiency:
A. Metabolic Factors
Fasting & Starvation: Increases beta oxidation as the body shifts to fat utilization.
Diet: High-fat diets induce beta oxidation, while high-carb diets inhibit it.
Exercise: Prolonged activity enhances beta oxidation for endurance performance.
B. Genetic Factors
Mutations in beta oxidation enzymes can lead to metabolic disorders.
Inherited defects in carnitine transport lead to lipid accumulation disorders.
C. Hormonal Influence
Thyroid hormones regulate metabolic rate, influencing the breakdown of fat
Cortisol promotes lipolysis during stress, activating beta oxidation.

Final Thoughts
Beta oxidation is a critical metabolic process that provides a high-energy yield from fatty acids. Hormones, enzyme activity, and cellular energy needs tightly regulate it—disruptions in beta oxidation lead to metabolic disorders, lipid storage diseases, and ketone imbalances.
A. Biological Significance
The primary energy source in fasting and exercise is when glucose levels drop.
Fatty acids provide sustained energy compared to carbohydrates.
Key pathway in endurance sports, prolonged fasting, and metabolic adaptation.
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
